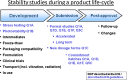
Stability studies needed to define the handling and transport conditions of sensitive pharmaceutical or biotechnological products
- PMID: 21948319
- PMCID: PMC3225534
- DOI: 10.1208/s12249-011-9684-0
Stability studies needed to define the handling and transport conditions of sensitive pharmaceutical or biotechnological products
Abstract
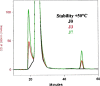
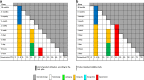
Many pharmaceutical or biotechnological products require transport using temperature-controlled systems to keep their therapeutic properties. There are presently no official guidelines for testing pharmaceutical products in order to define suitable transport specifications. After reviewing the current guidance documents, this paper proposes a methodology for testing pharmaceutical products and defining appropriate transport conditions.
Figures



References
-
- Stability testing of new drug substances and products—Q1A (R2). (ICH), International Conference on Harmonization. Originally published 1994, revised 2003.
-
- Stability testing: photostability testing of new drug substances and products—Q1B. (ICH), International Conference on Harmonization. 6 November 1996.
-
- Stability testing for new dosage forms—Q1C. (ICH), International Conference on Harmonization. 6 November 1996.
-
- Bracketing and matrixing designs for stability testing of new drug substances and products—Q1D. (ICH), International Conference on Harmonization. 7 February 2002. - PubMed
-
- Evaluation for stability data—Q1E. (ICH), International Conference on Harmonization. 6 February 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

