Human corneal endothelial cells employ phosphorylation of p27(Kip1) at both Ser10 and Thr187 sites for FGF-2-mediated cell proliferation via PI 3-kinase
- PMID: 21948550
- PMCID: PMC3208027
- DOI: 10.1167/iovs.11-8213
Human corneal endothelial cells employ phosphorylation of p27(Kip1) at both Ser10 and Thr187 sites for FGF-2-mediated cell proliferation via PI 3-kinase
Abstract
Purpose: FGF-2 stimulates cell proliferation of rabbit corneal endothelial cells (rCECs) by degrading the cyclin-dependent kinase inhibitor p27(Kip1) (p27) through its phosphorylation mechanism. The authors investigated whether the cell proliferation of human CECs (hCECs) is also induced by FGF-2 stimulation through the p27 phosphorylation pathway.
Methods: Expression and activation of protein were analyzed by immunoblotting. Cell proliferation was measured by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay. Transfection of hCECs with small interference RNA (siRNA) was performed using a transfection reagent.
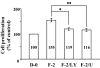
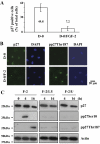
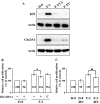
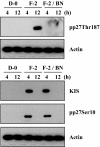
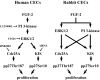
Results: FGF-2 stimulated cell proliferation in hCECs; the FGF-2 action was completely blocked by pathway-specific inhibitors for PI 3-kinase (LY294002) and MEK1/2 (U0126), respectively. Using immunoblotting, the authors showed that FGF-2 induced phosphorylation of p27 at both serine 10 (Ser10) and threonine 187 (Thr187) sites. These effects were also completely blocked by LY294002 or U0126. The authors then determined cross-talk between PI 3-kinase and extracellular signal-regulated kinase (ERK)1/2; blocking of ERK1/2 activation by LY294002 indicated that in hCECs ERK1/2 works as a downstream effector to PI 3-kinase for cell proliferation induced by FGF-2, whereas the ERK1/2 pathway in rCECs is parallel to the PI 3-kinase pathway. However, the downstream mechanism involved in cell cycle progression in hCECs is identical to that of rCECs: phosphorylation of p27 at Ser10 was mediated by kinase-interacting stathmin (KIS), confirmed with siRNA to KIS, and phosphorylation of p27 at Thr187 was mediated by cell division cycle 25A (Cdc25A), confirmed using Cdc25A inhibitor. CONCLUSIONS; FGF-2 stimulates proliferation of hCECs through PI 3-kinase and its downstream target ERK1/2 pathways. This linear signal transduction significantly downregulates p27 through its phosphorylation at both Ser10 and Thr187 sites mediated by KIS and Cdc25A, respectively.
Figures


References
-
- Kreutziger GO. Lateral membrane morphology and gap junction structure in rabbit corneal endothelium. Exp Eye Res. 1976;23:285–293 - PubMed
-
- Geroski DH, Edelhauser HF. Quantitation of Na/K ATPase pump sites in the rabbit corneal endothelium. Invest Ophthalmol Vis Sci. 1984;25:1056–1060 - PubMed
-
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22:359–389 - PubMed
-
- Joyce NC, Navon SE, Roy S, Zieske JD. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest Ophthalmol Vis Sci. 1996;37:1566–1575 - PubMed
-
- Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci. 2000;41:660–667 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

