Invariant NKT cell defects in vitamin D receptor knockout mice prevents experimental lung inflammation
- PMID: 21948983
- PMCID: PMC3197972
- DOI: 10.4049/jimmunol.1101519
Invariant NKT cell defects in vitamin D receptor knockout mice prevents experimental lung inflammation
Abstract
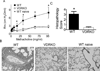
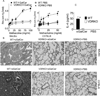
Vitamin D receptor (VDR) deficiency (knockout [KO]) results in a failure of mice to generate an airway hyperreactivity (AHR) response on both the BALB/c and C57BL/6 background. The cause of the failed AHR response is the defective population of invariant NKT (iNKT) cells in the VDR KO mice because wild-type (WT) iNKT cells rescued the AHR response. VDR KO mice had significantly fewer iNKT cells and normal numbers of T cells in the spleen compared with WT mice. In BALB/c VDR KO mice, the reduced frequencies of iNKT cells were not apparent in the liver or thymus. VDR KO and WT Th2 cells produced similar levels of IFN-γ and IL-5. On the BALB/c background, Th2 cells from VDR KO mice produced less IL-13, whereas on the C57BL/6 background, Th2 cells from VDR KO mice produced less IL-4. Conversely, VDR KO iNKT cells were defective for the production of multiple cytokines (BALB/c: IL-4, IL-5, and IL-13; C57BL/6: IL-4 and IL-17). Despite relatively normal Th2 responses, BALB/c and C57BL/6 VDR KO mice failed to develop AHR responses. The defect in iNKT cells as a result of the VDR KO was more important than the highly susceptible Th2 background of the BALB/c mice. Defective iNKT cell responses in the absence of the VDR result in the failure to generate AHR responses in the lung. The implication of these mechanistic findings for human asthma requires further investigation.
Figures



References
-
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. - PubMed
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
-
- Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. - PubMed
-
- Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–393. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

