Tropodithietic acid production in Phaeobacter gallaeciensis is regulated by N-acyl homoserine lactone-mediated quorum sensing
- PMID: 21949069
- PMCID: PMC3232910
- DOI: 10.1128/JB.05818-11
Tropodithietic acid production in Phaeobacter gallaeciensis is regulated by N-acyl homoserine lactone-mediated quorum sensing
Abstract
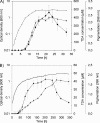
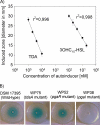
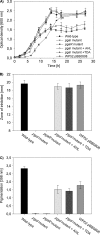
The production of N-acyl homoserine lactones (AHLs) is widely distributed within the marine Roseobacter clade, and it was proposed that AHL-mediated quorum sensing (QS) is one of the most common cell-to-cell communication mechanisms in roseobacters. The traits regulated by AHL-mediated QS are yet not known for members of the Roseobacter clade, but production of the antibiotic tropodithietic acid (TDA) was supposed to be controlled by AHL-mediated QS in Phaeobacter spp. We describe here for the first time the functional role of luxR and luxI homologous genes of an organism of the Roseobacter clade, i.e., pgaR and pgaI in Phaeobacter gallaeciensis. Our results demonstrate that the AHL synthase gene pgaI is responsible for production of N-3-hydroxydecanoylhomoserine lactone (3OHC(10)-HSL). Insertion mutants of pgaI and pgaR are both deficient in TDA biosynthesis and the formation of a yellow-brown pigment when grown in liquid marine broth medium. This indicates that in P. gallaeciensis the production of both secondary metabolites is controlled by AHL-mediated QS. Quantitative real-time PCR showed that the transcription level of tdaA, which encodes an essential transcriptional regulator for TDA biosynthesis, decreased 28- and 51-fold in pgaI and pgaR genetic backgrounds, respectively. These results suggest that both the response regulator PgaR and the 3OHC(10)-HSL produced by PgaI induce expression of tdaA, which in turn positively regulates expression of the tda genes. Moreover, we confirmed that TDA can also act as autoinducer in P. gallaeciensis, as previously described for Silicibacter sp. strain TM1040, but only in the presence of the response regulator PgaR.
Figures


Similar articles
-
Phaeobacter sp. strain Y4I utilizes two separate cell-to-cell communication systems to regulate production of the antimicrobial indigoidine.Appl Environ Microbiol. 2015 Feb;81(4):1417-25. doi: 10.1128/AEM.02551-14. Appl Environ Microbiol. 2015. PMID: 25527537 Free PMC article.
-
Genetic dissection of tropodithietic acid biosynthesis by marine roseobacters.Appl Environ Microbiol. 2008 Mar;74(5):1535-45. doi: 10.1128/AEM.02339-07. Epub 2008 Jan 11. Appl Environ Microbiol. 2008. PMID: 18192410 Free PMC article.
-
Expression of tropodithietic acid biosynthesis is controlled by a novel autoinducer.J Bacteriol. 2010 Sep;192(17):4377-87. doi: 10.1128/JB.00410-10. Epub 2010 Jul 2. J Bacteriol. 2010. PMID: 20601479 Free PMC article.
-
Acyl-homoserine lactone quorum sensing in the Roseobacter clade.Int J Mol Sci. 2014 Jan 7;15(1):654-69. doi: 10.3390/ijms15010654. Int J Mol Sci. 2014. PMID: 24402124 Free PMC article. Review.
-
An evolving perspective on the Pseudomonas aeruginosa orphan quorum sensing regulator QscR.Front Cell Infect Microbiol. 2014 Oct 28;4:152. doi: 10.3389/fcimb.2014.00152. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25389523 Free PMC article. Review.
Cited by
-
Changes in the Structure of the Microbial Community Associated with Nannochloropsis salina following Treatments with Antibiotics and Bioactive Compounds.Front Microbiol. 2016 Jul 26;7:1155. doi: 10.3389/fmicb.2016.01155. eCollection 2016. Front Microbiol. 2016. PMID: 27507966 Free PMC article.
-
Microbial Surface Colonization and Biofilm Development in Marine Environments.Microbiol Mol Biol Rev. 2015 Dec 23;80(1):91-138. doi: 10.1128/MMBR.00037-15. Print 2016 Mar. Microbiol Mol Biol Rev. 2015. PMID: 26700108 Free PMC article. Review.
-
Pearl Oyster Bacterial Community Structure Is Governed by Location and Tissue-Type, but Vibrio Species Are Shared Among Oyster Tissues.Front Microbiol. 2021 Aug 9;12:723649. doi: 10.3389/fmicb.2021.723649. eCollection 2021. Front Microbiol. 2021. PMID: 34434182 Free PMC article.
-
Contributions of tropodithietic acid and biofilm formation to the probiotic activity of Phaeobacter inhibens.BMC Microbiol. 2016 Jan 5;16:1. doi: 10.1186/s12866-015-0617-z. BMC Microbiol. 2016. PMID: 26728027 Free PMC article.
-
Assessing the exoproteome of marine bacteria, lesson from a RTX-toxin abundantly secreted by Phaeobacter strain DSM 17395.PLoS One. 2014 Feb 24;9(2):e89691. doi: 10.1371/journal.pone.0089691. eCollection 2014. PLoS One. 2014. PMID: 24586966 Free PMC article.
References
-
- Atkinson S., Sockett R. E., Camara M., Williams P. 2006. Quorum sensing and the lifestyle of Yersinia. Curr. Issues Mol. Biol. 8:1–10 - PubMed
-
- Bauer W. D., Mathesius U. 2004. Plant responses to bacterial quorum sensing signals. Curr. Opin. Plant Biol. 7:429–433 - PubMed
-
- Bibb M. 1996. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 142:1335–1344 - PubMed
-
- Boyer M., Wisniewski-Dye F. 2009. Cell-cell signaling in bacteria: not simply a matter of quorum. FEMS Microbiol. Ecol. 70:1–19 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

