Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation
- PMID: 21949096
- PMCID: PMC3280000
- DOI: 10.1681/ASN.2010111160
Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation
Abstract
Calcineurin-inhibitor-sparing strategies in kidney transplantation may spare patients the adverse effects of these drugs, but the efficacy of these strategies is unknown. Here, we conduct a meta-analysis to assess outcomes associated with reducing calcineurin inhibitor exposure from the time of transplantation. We search Medline, Embase, and Cochrane Register of Controlled Trials for randomized controlled trials published between 1966 and 2010 that compared de novo calcineurin-inhibitor-sparing regimens to calcineurin-inhibitor-based regimens. In this analysis, we include 56 studies comprising data from 11337 renal transplant recipients. Use of the contemporary agents belatacept or tofacitinib, in combination with mycophenolate, decreased the odds of overall graft failure (OR 0.61; 95% CI 0.39-0.96; P = 0.03). Similarly, minimization of calcineurin inhibitors in combination with various induction and adjunctive agents reduces the odds of graft failure (OR 0.73; 95% CI 0.58-0.92; P = 0.009). Conversely, the use of inhibitors of mammalian target of rapamycin (mTOR), in combination with mycophenolate, increases the odds of graft failure (OR 1.43; 95% CI 1.08-1.90; P = 0.01). Calcineurin-inhibitor-sparing strategies are associated with less delayed graft function (OR 0.89; 95% CI 0.80-0.98; P = 0.02), improved graft function, and less new-onset diabetes. The more contemporary protocols did not seem to increase rates of acute rejection. In conclusion, this meta-analysis suggests that reducing exposure to calcineurin inhibitors immediately after kidney transplantation may improve clinical outcomes.
Figures
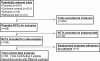
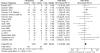
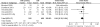
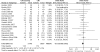


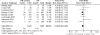
References
-
- Borel JF, Kis ZL: The discovery and development of cyclosporine (Sandimmune). Transplant Proc 23: 1867–74, 1991 - PubMed
-
- Calne RY, Thiru S, Mcmaster P, Craddock GN, White DJG, Evans DB, Dunn DC, Pentlow BD, Rolles K: Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 2: 1323–1327, 1978 - PubMed
-
- A randomized clinical trial of cyclosporine in cadaveric renal transplantation. N Engl J Med 309: 809–815, 1983 - PubMed
-
- Group EMT: Cyclosporin in cadaveric renal transplantation: One-year follow-up of a multicentre trial. The Lancet 2: 986–989, 1983 - PubMed
-
- McMaster P, Haynes IG, Michael J, Adu D, Vlassis T, Roger S, Turney J, Stock S, Buckels J, Mackintosh P, Ezzibdeh M: Cyclosporine in cadaveric renal transplantation: A prospective randomized trial. Transplant Proc 15: 2523–2527, 1983
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

