HPV and HPV vaccine education intervention: effects on parents, healthcare staff, and school staff
- PMID: 21949110
- PMCID: PMC3210879
- DOI: 10.1158/1055-9965.EPI-11-0562
HPV and HPV vaccine education intervention: effects on parents, healthcare staff, and school staff
Abstract
Background: Increasing knowledge about human papillomavirus (HPV) and HPV vaccine is a potentially important way to increase vaccination rates, yet few education interventions have addressed these topics. We report the results of an education intervention targeting three key groups who have contact with adolescent females.
Methods: We conducted HPV education intervention sessions during 2008 and 2009 in Guilford County, North Carolina. Parents (n = 376), healthcare staff (n = 118), and school staff (n = 456) attended the one-time sessions and completed self-administered surveys. Analyses used mixed regression models to examine the intervention's effects on participants' self-rated HPV knowledge, objectively assessed HPV and HPV vaccine knowledge, and beliefs about HPV vaccine.
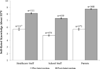
Results: Participants had relatively low levels of objectively assessed HPV and HPV vaccine knowledge prior to the intervention. The education intervention increased self-rated HPV knowledge among all three key groups (all P < 0.001), and objectively assessed knowledge about many aspects of HPV and HPV vaccine among healthcare and school staff members (all P < 0.05). Following the intervention, more than 90% of school staff members believed HPV and HPV vaccine education is worthwhile for school personnel and that middle schools are an appropriate venue for this education. Most parents (97%) and school staff members (85%) indicated they would be supportive of school-based vaccination clinics.
Conclusions: Our education intervention greatly increased HPV and HPV vaccine knowledge among groups influential to the HPV vaccination behaviors of adolescent females.
Impact: Education interventions represent a simple yet potentially effective strategy for increasing HPV vaccination and garnering stronger support for school-based vaccination clinics.
© 2011 AACR.
Figures
References
-
- Centers for Disease Control and Prevention (CDC) FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):626–629. - PubMed
-
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–632. - PubMed
-
- U.S. Food and Drug Administration. December 22, 2010 approval letter - Gardasil. Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm2....
-
- Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13–17 years --- United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(33):1117–1123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources