Different signaling pathways stimulate a disintegrin and metalloprotease-17 (ADAM17) in neutrophils during apoptosis and activation
- PMID: 21949123
- PMCID: PMC3234723
- DOI: 10.1074/jbc.M111.277087
Different signaling pathways stimulate a disintegrin and metalloprotease-17 (ADAM17) in neutrophils during apoptosis and activation
Abstract
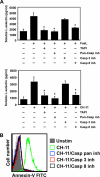
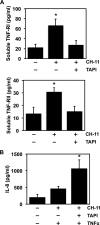
ADAM17 is a membrane-associated metalloprotease that cleaves proteins from the surface of neutrophils and modulates the density of various receptors and adhesion molecules. The protease activity of ADAM17 is highly inducible and occurs upon neutrophil activation as well as apoptosis. At this time, little is known about the signal transduction pathway that promotes ADAM17 activity in neutrophils upon the induction of apoptosis. We show that caspase-8 activation, Bid cleavage, and the release of mitochondrial reactive oxygen species are sequential transduction components of the Fas signaling cascade that induces ADAM17. This is different from ADAM17 stimulation upon overt neutrophil activation, which requires MAPK p38 or ERK, but not caspases and reactive oxygen species. ADAM17 activity in apoptotic neutrophils may serve to inactivate select effector molecules that promote the pro-inflammatory activity of recruited neutrophils. For instance, TNFα receptors TNF-RI and TNF-RII are substrates of ADAM17, and we show that they are shed during apoptosis, decreasing neutrophil sensitivity to TNFα. Altogether, our findings provide significant new insights into the signal transduction pathway that stimulates ADAM17 during induced neutrophil apoptosis. ADAM17 induction during apoptosis may rapidly diminish neutrophil sensitivity to the inflammatory environment, complementing other anti-inflammatory activities by these cells during inflammation resolution.
Figures





References
-
- Reiss K., Saftig P. (2009) Semin. Cell Dev. Biol. 20, 126–137 - PubMed
-
- Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S., Nelson N., Boiani N., Schooley K. A., Gerhart M., Davis R., Fitzner J. N., Johnson R. S., Paxton R. J., March C. J., Cerretti D. P. (1997) Nature 385, 729–733 - PubMed
-
- Moss M. L., Jin S. L., Milla M. E., Bickett D. M., Burkhart W., Carter H. L., Chen W. J., Clay W. C., Didsbury J. R., Hassler D., Hoffman C. R., Kost T. A., Lambert M. H., Leesnitzer M. A., McCauley P., McGeehan G., Mitchell J., Moyer M., Pahel G., Rocque W., Overton L. K., Schoenen F., Seaton T., Su J. L., Becherer J. D. (1997) Nature 385, 733–736 - PubMed
-
- Arribas J., Esselens C. (2009) Curr. Pharm. Des. 15, 2319–2335 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

