TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc
- PMID: 21949132
- PMCID: PMC3220535
- DOI: 10.1074/jbc.M111.264549
TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc
Abstract
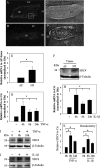
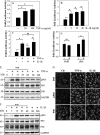
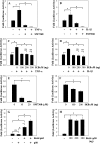
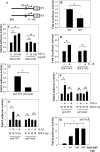
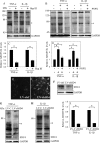
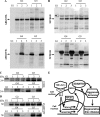
Elevated levels of TNF-α, IL-1β and a resultant increase in ADAMTS (a disintegrin-like and metalloprotease with thrombospondin type I motifs) expression is seen during disc degeneration. However, if these pro-inflammatory cytokines control ADAMTS activity is not definitively known. The goal of the investigation was to study if TNF-α and IL-1β regulate syndecan-4 (SDC4) expression, and if SDC4 was responsible for promoting aggrecan degradation through controlling ADAMTS activity in nucleus pulposus cells of the intervertebral disc. Cytokine treatment increased SDC4 expression and promoter activity. Use of inhibitor, SM7368 and co-transfections with IκBα, RelA/p50 showed that NF-κΒ regulated both basal and cytokine-dependent SDC4 transcription. SDC4 promoter harboring RelA binding site mutation was unresponsive to the cytokines. Moreover, cytokines failed to increase SDC4 promoter activity in RelA-null cells. Cytokines increased ADAMTS-4/5 expression and aggrecan degradation and promoted SDC4 interaction with ADAMTS-5. Treatment with heparinase-III and p-nitrophenyl-β-D-xylopyranoside (PNPX), an inhibitor of heparan sulfate synthesis and transfection with SDC4-shRNA partially blocked cytokine mediated aggrecan degradation. Analysis of human tissues showed increased aggrecan degradation with a concomitant increase in SDC4 and ADAMTS-5 protein expression with severity of disc disease. Likewise, SDC4, TNF-α, IL-1β, ADAMTS-4, and ADAMTS-5 mRNA expression increased in degenerate tissues. We conclude that in nucleus pulposus, TNF-α and IL-1β regulate SDC4 expression, which plays a key role in pathogenesis of degenerative disc disease by promoting aggrecan degradation by ADAMTS-5.
Figures


References
-
- Feng H., Danfelter M., Stromqvist B., Heinegard D. (2006) J Bone Joint Surg Am 88, Suppl. 2, 25–29 - PubMed
-
- Setton L.A., Chen J. (2006) J. Bone Joint Surg. Am. 88, Suppl. 2, 52–57 - PubMed
-
- Ng L, Grodzinsky A.J., Patwari P., Sandy J., Plaas A., Ortiz C. (2003) J. Struct. Biol. 143, 242–257 - PubMed
-
- Roberts S., Evans H., Trivedi J., Menage J. (2006) J. Bone Joint Surg. Am. 88, Suppl. 2, 10–14 - PubMed
-
- Miljkovic D., Trajkovic V. (2004) Cytokine Growth Factor Rev. 15, 21–32 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

