Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters
- PMID: 21949165
- PMCID: PMC3960856
- DOI: 10.1001/archinternmed.2011.401
Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters
Abstract
Background: To estimate the clinical benefit of highly active antiretroviral therapy (HAART) initiation vs deferral in a given month in patients with CD4 cell counts less than 800/μL.
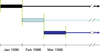
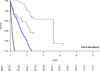
Methods: In this observational cohort study of human immunodeficiency virus type 1 seroconverters from CASCADE (Concerted Action on SeroConversion to AIDS and Death in Europe), we constructed monthly sequential nested subcohorts between January 1996 and May 2009, including all eligible HAART-naive, AIDS-free individuals with a CD4 cell count less than 800/μL. The primary outcome was time to AIDS or death in those who initiated HAART in the baseline month compared with those who did not, pooled across subcohorts and stratified by CD4 cell count. Using inverse probability-of-treatment weighted survival curves and Cox proportional hazards regression models, we estimated the absolute and relative effects of treatment with robust 95% confidence intervals (CIs).
Results: Of 9455 patients with 52,268 person-years of follow-up, 812 (8.6%) developed AIDS and 544 (5.8%) died. In CD4 cell count strata of 200 to 349, 350 to 499, and 500 to 799/μL, HAART initiation was associated with adjusted hazard ratios (95% CIs) for AIDS/death of 0.59 (0.43-0.81), 0.75 (0.49-1.14), and 1.10 (0.67-1.79), respectively. In the analysis of all-cause mortality, HAART initiation was associated with adjusted hazard ratios (95% CIs) of 0.71 (0.44-1.15), 0.51 (0.33-0.80), and 1.02 (0.49-2.12), respectively. Numbers needed to treat (95% CIs) to prevent 1 AIDS event or death within 3 years were 21 (14-38) and 34 (20-115) in CD4 cell count strata of 200 to 349 and 350 to 499/μL, respectively.
Conclusion: Compared with deferring in a given month, HAART initiation at CD4 cell counts less than 500/μL (but not 500-799/μL) was associated with slower disease progression.
Conflict of interest statement
The following authors have no conflicts of interest to report: KEH, JCT, JSK and MD.
Figures





Comment in
-
HAART for HIV-1 infection: zeroing in on when to start: comment on "Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters".Arch Intern Med. 2011 Sep 26;171(17):1569-70. doi: 10.1001/archinternmed.2011.402. Arch Intern Med. 2011. PMID: 21949166 No abstract available.
References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. - PubMed
-
- Cameron DW, Heath-Chiozzi M, Danner S, et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet. 1998 Feb 21;351(9102):543–549. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997 Sep 11;337(11):725–733. - PubMed
-
- Strategic Timing of Antiretroviral Treatment (START) - ClinicalTrials.gov. National Institutes of Health. 2010 Jan 30; Available from: http://clinicaltrials.gov/ct2/show/NCT00867048.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

