Tyrosine aminotransferase contributes to benzylisoquinoline alkaloid biosynthesis in opium poppy
- PMID: 21949209
- PMCID: PMC3252151
- DOI: 10.1104/pp.111.185512
Tyrosine aminotransferase contributes to benzylisoquinoline alkaloid biosynthesis in opium poppy
Abstract
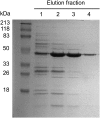
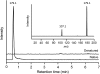
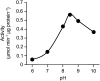
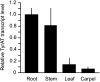
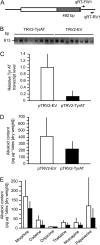
Tyrosine aminotransferase (TyrAT) catalyzes the transamination of L-Tyr and α-ketoglutarate, yielding 4-hydroxyphenylpyruvic acid and L-glutamate. The decarboxylation product of 4-hydroxyphenylpyruvic acid, 4-hydroxyphenylacetaldehyde, is a precursor to a large and diverse group of natural products known collectively as benzylisoquinoline alkaloids (BIAs). We have isolated and characterized a TyrAT cDNA from opium poppy (Papaver somniferum), which remains the only commercial source for several pharmaceutical BIAs, including codeine, morphine, and noscapine. TyrAT belongs to group I pyridoxal 5'-phosphate (PLP)-dependent enzymes wherein Schiff base formation occurs between PLP and a specific Lys residue. The amino acid sequence of TyrAT showed considerable homology to other putative plant TyrATs, although few of these have been functionally characterized. Purified, recombinant TyrAT displayed a molecular mass of approximately 46 kD and a substrate preference for L-Tyr and α-ketoglutarate, with apparent K(m) values of 1.82 and 0.35 mm, respectively. No specific requirement for PLP was detected in vitro. Liquid chromatography-tandem mass spectrometry confirmed the conversion of L-Tyr to 4-hydroxyphenylpyruvate. TyrAT gene transcripts were most abundant in roots and stems of mature opium poppy plants. Virus-induced gene silencing was used to evaluate the contribution of TyrAT to BIA metabolism in opium poppy. TyrAT transcript levels were reduced by at least 80% in silenced plants compared with controls and showed a moderate reduction in total alkaloid content. The modest correlation between transcript levels and BIA accumulation in opium poppy supports a role for TyrAT in the generation of alkaloid precursors, but it also suggests the occurrence of other sources for 4-hydroxyphenylacetaldehyde.
Figures
References
-
- Adcock HJ, Gaskin PJ, Shaw PN, Teesdale-Spittle PH, Buckberry LD. (1996) Novel sources of mammalian C-S lyase activity. J Pharm Pharmacol 48: 150–153 - PubMed
-
- Andersson SM, Pispa JP. (1982) Purification and properties of human liver tyrosine aminotransferase. Clin Chim Acta 125: 117–123 - PubMed
-
- Antognoni F, Faudale M, Poli F, Biondi S. (2009) Methyl jasmonate differentially affects tocopherol content and tyrosine amino transferase activity in cultured cells of Amaranthus caudatus and Chenopodium quinoa. Plant Biol (Stuttg) 11: 161–169 - PubMed
-
- Barta IC, Böger P. (1996) Purification and characterization of 4-hydroxyphenylpyruvate dioxygenase from maize. Pestic Sci 48: 109–116
-
- Bein NN, Goldsmith HS. (1977) Recurrent massive haemorrhage from benign hepatic tumours secondary to oral contraceptives. Br J Surg 64: 433–435 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

