Phosphomannose isomerase inhibitors improve N-glycosylation in selected phosphomannomutase-deficient fibroblasts
- PMID: 21949237
- PMCID: PMC3234766
- DOI: 10.1074/jbc.M111.285502
Phosphomannose isomerase inhibitors improve N-glycosylation in selected phosphomannomutase-deficient fibroblasts
Erratum in
- J Biol Chem. 2011 Dec 16;286(50):43588. Scott, David A [added]
Abstract
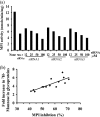
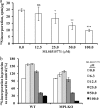
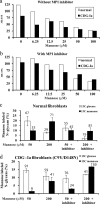
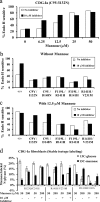
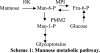
Congenital disorders of glycosylation (CDG) are rare genetic disorders due to impaired glycosylation. The patients with subtypes CDG-Ia and CDG-Ib have mutations in the genes encoding phosphomannomutase 2 (PMM2) and phosphomannose isomerase (MPI or PMI), respectively. PMM2 (mannose 6-phosphate → mannose 1-phosphate) and MPI (mannose 6-phosphate ⇔ fructose 6-phosphate) deficiencies reduce the metabolic flux of mannose 6-phosphate (Man-6-P) into glycosylation, resulting in unoccupied N-glycosylation sites. Both PMM2 and MPI compete for the same substrate, Man-6-P. Daily mannose doses reverse most of the symptoms of MPI-deficient CDG-Ib patients. However, CDG-Ia patients do not benefit from mannose supplementation because >95% Man-6-P is catabolized by MPI. We hypothesized that inhibiting MPI enzymatic activity would provide more Man-6-P for glycosylation and possibly benefit CDG-Ia patients with residual PMM2 activity. Here we show that MLS0315771, a potent MPI inhibitor from the benzoisothiazolone series, diverts Man-6-P toward glycosylation in various cell lines including fibroblasts from CDG-Ia patients and improves N-glycosylation. Finally, we show that MLS0315771 increases mannose metabolic flux toward glycosylation in zebrafish embryos.
Figures



Similar articles
-
A zebrafish model of PMM2-CDG reveals altered neurogenesis and a substrate-accumulation mechanism for N-linked glycosylation deficiency.Mol Biol Cell. 2012 Nov;23(21):4175-87. doi: 10.1091/mbc.E12-05-0411. Epub 2012 Sep 5. Mol Biol Cell. 2012. PMID: 22956764 Free PMC article.
-
A zebrafish model of congenital disorders of glycosylation with phosphomannose isomerase deficiency reveals an early opportunity for corrective mannose supplementation.Dis Model Mech. 2013 Jan;6(1):95-105. doi: 10.1242/dmm.010116. Epub 2012 Aug 16. Dis Model Mech. 2013. PMID: 22899857 Free PMC article.
-
Liposome-encapsulated mannose-1-phosphate therapy improves global N-glycosylation in different congenital disorders of glycosylation.Mol Genet Metab. 2024 Jun;142(2):108487. doi: 10.1016/j.ymgme.2024.108487. Epub 2024 May 7. Mol Genet Metab. 2024. PMID: 38733638 Free PMC article.
-
New and potential strategies for the treatment of PMM2-CDG.Biochim Biophys Acta Gen Subj. 2020 Nov;1864(11):129686. doi: 10.1016/j.bbagen.2020.129686. Epub 2020 Jul 23. Biochim Biophys Acta Gen Subj. 2020. PMID: 32712172 Review.
-
Carbohydrate-deficient glycoprotein syndrome type IA (phosphomannomutase-deficiency).Biochim Biophys Acta. 1999 Oct 8;1455(2-3):155-65. doi: 10.1016/s0925-4439(99)00073-3. Biochim Biophys Acta. 1999. PMID: 10571009 Review.
Cited by
-
Yeast Models of Phosphomannomutase 2 Deficiency, a Congenital Disorder of Glycosylation.G3 (Bethesda). 2019 Feb 7;9(2):413-423. doi: 10.1534/g3.118.200934. G3 (Bethesda). 2019. PMID: 30530630 Free PMC article.
-
Study of global transcriptional changes of N-GlcNAc2 proteins-producing T24 bladder carcinoma cells under glucose deprivation.PLoS One. 2013;8(4):e60397. doi: 10.1371/journal.pone.0060397. Epub 2013 Apr 1. PLoS One. 2013. PMID: 23560094 Free PMC article.
-
Dietary mannose supplementation in phosphomannomutase 2 deficiency (PMM2-CDG).Orphanet J Rare Dis. 2020 Sep 22;15(1):258. doi: 10.1186/s13023-020-01528-z. Orphanet J Rare Dis. 2020. PMID: 32962735 Free PMC article.
-
Congenital disorders of glycosylation with neonatal presentation.BMJ Case Rep. 2014 Apr 16;2014:bcr2013010037. doi: 10.1136/bcr-2013-010037. BMJ Case Rep. 2014. PMID: 24739649 Free PMC article.
-
CDG Therapies: From Bench to Bedside.Int J Mol Sci. 2018 Apr 27;19(5):1304. doi: 10.3390/ijms19051304. Int J Mol Sci. 2018. PMID: 29702557 Free PMC article. Review.
References
-
- Van Schaftingen E., Jaeken J. (1995) FEBS Lett. 377, 318–320 - PubMed
-
- Pirard M., Matthijs G., Heykants L., Schollen E., Grünewald S., Jaeken J., van Schaftingen E. (1999) FEBS Lett. 452, 319–322 - PubMed
-
- Grünewald S. (2009) Biochim. Biophys. Acta 1792, 827–834 - PubMed
-
- Freeze H. H. (2006) Nat. Rev. Genet. 7, 537–551 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

