Rumi functions as both a protein O-glucosyltransferase and a protein O-xylosyltransferase
- PMID: 21949356
- PMCID: PMC3189016
- DOI: 10.1073/pnas.1109696108
Rumi functions as both a protein O-glucosyltransferase and a protein O-xylosyltransferase
Abstract
Mutations in rumi result in a temperature-sensitive loss of Notch signaling in Drosophila. Drosophila Rumi is a soluble, endoplasmic reticulum-retained protein with a CAP10 domain that functions as a protein O-glucosyltransferase. In human and mouse genomes, three potential Rumi homologues exist: one with a high degree of identity to Drosophila Rumi (52%), and two others with lower degrees of identity but including a CAP10 domain (KDELC1 and KDELC2). Here we show that both mouse and human Rumi, but not KDELC1 or KDELC2, catalyze transfer of glucose from UDP-glucose to an EGF repeat from human factor VII. Similarly, human Rumi, but not KDELC1 or KDELC2, rescues the Notch phenotypes in Drosophila rumi clones. During characterization of the Rumi enzymes, we noted that, in addition to protein O-glucosyltransferase activity, both mammalian and Drosophila Rumi also showed significant protein O-xylosyltransferase activity. Rumi transfers Xyl or glucose to serine 52 in the O-glucose consensus sequence ( ) of factor VII EGF repeat. Surprisingly, the second serine (S53) facilitates transfer of Xyl, but not glucose, to the EGF repeat by Rumi. EGF16 of mouse Notch2, which has a diserine motif in the consensus sequence ( ), is also modified with either O-Xyl or O-glucose glycans in cells. Mutation of the second serine (S590A) causes a loss of O-Xyl but not O-glucose at this site. Altogether, our data establish dual substrate specificity for the glycosyltransferase Rumi and provide evidence that amino acid sequences of the recipient EGF repeat significantly influence which donor substrate (UDP-glucose or UDP-Xyl) is used.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

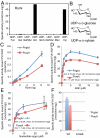


 from wild-type mNotch2 EGF16. The MS data were searched for the doubly charged form of these (glyco)peptides: m/z 801.0 for the unglycosylated, m/z 1,014.0 for the O-glucosylated, and m/z 999.0 for the O-xylosylated forms. (B) EICs of the ions corresponding to the unglycosylated (Top), O-glucosylated (Middle), and O-xylosylated (Bottom) forms of
from wild-type mNotch2 EGF16. The MS data were searched for the doubly charged form of these (glyco)peptides: m/z 801.0 for the unglycosylated, m/z 1,014.0 for the O-glucosylated, and m/z 999.0 for the O-xylosylated forms. (B) EICs of the ions corresponding to the unglycosylated (Top), O-glucosylated (Middle), and O-xylosylated (Bottom) forms of  from the mutated mNotch2 EGF16. The MS data were searched for the doubly charged form of these (glyco)peptides: m/z 793.0 for the unglycosylated (Top), m/z 1,006.0 for the O-glucosylated (Middle), and m/z 991.0 for the O-xylosylated (Bottom) forms.
from the mutated mNotch2 EGF16. The MS data were searched for the doubly charged form of these (glyco)peptides: m/z 793.0 for the unglycosylated (Top), m/z 1,006.0 for the O-glucosylated (Middle), and m/z 991.0 for the O-xylosylated (Bottom) forms.Similar articles
-
Molecular cloning of a xylosyltransferase that transfers the second xylose to O-glucosylated epidermal growth factor repeats of notch.J Biol Chem. 2012 Jan 20;287(4):2739-48. doi: 10.1074/jbc.M111.302406. Epub 2011 Nov 23. J Biol Chem. 2012. PMID: 22117070 Free PMC article.
-
Negative regulation of notch signaling by xylose.PLoS Genet. 2013 Jun;9(6):e1003547. doi: 10.1371/journal.pgen.1003547. Epub 2013 Jun 6. PLoS Genet. 2013. PMID: 23754965 Free PMC article.
-
Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling.Cell. 2008 Jan 25;132(2):247-58. doi: 10.1016/j.cell.2007.12.016. Cell. 2008. PMID: 18243100 Free PMC article.
-
Regulation of notch signaling via O-glucosylation insights from Drosophila studies.Methods Enzymol. 2010;480:375-98. doi: 10.1016/S0076-6879(10)80017-5. Methods Enzymol. 2010. PMID: 20816218 Review.
-
Structure, function, and pathology of protein O-glucosyltransferases.Cell Death Dis. 2021 Jan 12;12(1):71. doi: 10.1038/s41419-020-03314-y. Cell Death Dis. 2021. PMID: 33436558 Free PMC article. Review.
Cited by
-
A comprehensive role evaluation and mechanism exploration of POGLUT2 in pan-cancer.Front Oncol. 2022 Sep 8;12:962540. doi: 10.3389/fonc.2022.962540. eCollection 2022. Front Oncol. 2022. PMID: 36158688 Free PMC article.
-
Structural analysis of Notch-regulating Rumi reveals basis for pathogenic mutations.Nat Chem Biol. 2016 Sep;12(9):735-40. doi: 10.1038/nchembio.2135. Epub 2016 Jul 18. Nat Chem Biol. 2016. PMID: 27428513 Free PMC article.
-
Dual Roles of O-Glucose Glycans Redundant with Monosaccharide O-Fucose on Notch in Notch Trafficking.J Biol Chem. 2016 Jun 24;291(26):13743-52. doi: 10.1074/jbc.M115.710483. Epub 2016 Apr 25. J Biol Chem. 2016. PMID: 27129198 Free PMC article.
-
Glycosyltransferase genes that cause monogenic congenital disorders of glycosylation are distinct from glycosyltransferase genes associated with complex diseases.Glycobiology. 2018 May 1;28(5):284-294. doi: 10.1093/glycob/cwy015. Glycobiology. 2018. PMID: 29579191 Free PMC article.
-
O-fucose monosaccharide of Drosophila Notch has a temperature-sensitive function and cooperates with O-glucose glycan in Notch transport and Notch signaling activation.J Biol Chem. 2015 Jan 2;290(1):505-19. doi: 10.1074/jbc.M114.616847. Epub 2014 Nov 5. J Biol Chem. 2015. PMID: 25378397 Free PMC article.
References
-
- Fortini ME. Notch signaling: The core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. - PubMed
-
- Rampal R, Luther KB, Haltiwanger RS. Notch signaling in normal and disease states: Possible therapies related to glycosylation. Curr Mol Med. 2007;7:427–445. - PubMed
-
- Moloney DJ, et al. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J Biol Chem. 2000;275:9604–9611. - PubMed
-
- Matsuura A, et al. O-linked N-acetylglucosamine is present on the extracellular domain of notch receptors. J Biol Chem. 2008;283:35486–35495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

