Nuclear receptor liver receptor homologue 1 (LRH-1) regulates pancreatic cancer cell growth and proliferation
- PMID: 21949357
- PMCID: PMC3193228
- DOI: 10.1073/pnas.1112047108
Nuclear receptor liver receptor homologue 1 (LRH-1) regulates pancreatic cancer cell growth and proliferation
Abstract
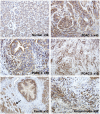
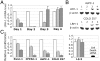
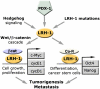
An essential regulator of gene transcription, nuclear receptor liver receptor homologue 1 (LRH-1) controls cell differentiation in the developing pancreas and maintains cholesterol homeostasis in adults. Recent genome-wide association studies linked mutations in the LRH-1 gene and its up-stream regulatory regions to development of pancreatic cancer. In this work, we show that LRH-1 transcription is activated up to 30-fold in human pancreatic cancer cells compared to normal pancreatic ductal epithelium. This activation correlates with markedly increased LRH-1 protein expression in human pancreatic ductal adenocarcinomas in vivo. Selective blocking of LRH-1 by receptor specific siRNA significantly inhibits pancreatic cancer cell proliferation in vitro. The inhibition is tracked in part to the attenuation of the receptor's transcriptional targets controlling cell growth, proliferation, and differentiation. Previously, LRH-1 was shown to contribute to formation of intestinal tumors. This study demonstrates the critical involvement of LRH-1 in development and progression of pancreatic cancer, suggesting the LRH-1 receptor as a plausible therapeutic target for treatment of pancreatic ductal adenocarcinomas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

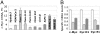
References
-
- Fayard E, Auwerx J, Schoonjans K. LRH-1: An orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14:250–260. - PubMed
-
- Fayard E, Schoonjans K, Annicotte J-S, Auwerx J. Liver receptor homolog 1 controls the expression of carboxyl ester lipase. J Biol Chem. 2003;278:35725–35731. - PubMed
-
- Kim JW, Peng N, Rainey WE, Carr BR, Attia GR. Liver receptor homolog-1 regulates the expression of steroidogenic acute regulatory protein in human granulosa cells. J Clin Endocrinol Metab. 2004;89:3042–3047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

