Direct measurement of the protein response to an electrostatic perturbation that mimics the catalytic cycle in ketosteroid isomerase
- PMID: 21949360
- PMCID: PMC3189056
- DOI: 10.1073/pnas.1113874108
Direct measurement of the protein response to an electrostatic perturbation that mimics the catalytic cycle in ketosteroid isomerase
Abstract
Understanding how electric fields and their fluctuations in the active site of enzymes affect efficient catalysis represents a critical objective of biochemical research. We have directly measured the dynamics of the electric field in the active site of a highly proficient enzyme, Δ(5)-3-ketosteroid isomerase (KSI), in response to a sudden electrostatic perturbation that simulates the charge displacement that occurs along the KSI catalytic reaction coordinate. Photoexcitation of a fluorescent analog (coumarin 183) of the reaction intermediate mimics the change in charge distribution that occurs between the reactant and intermediate state in the steroid substrate of KSI. We measured the electrostatic response and angular dynamics of four probe dipoles in the enzyme active site by monitoring the time-resolved changes in the vibrational absorbance (IR) spectrum of a spectator thiocyanate moiety (a quantitative sensor of changes in electric field) placed at four different locations in and around the active site, using polarization-dependent transient vibrational Stark spectroscopy. The four different dipoles in the active site remain immobile and do not align to the changes in the substrate electric field. These results indicate that the active site of KSI is preorganized with respect to functionally relevant changes in electric fields.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
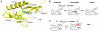
 ) of approximately 20.2 debye is created from the movement of a single electronic charge over a distance of 4.2 Å during the passage from KSI–intermediate complex to KSI–substrate complex, which is similar in magnitude to
) of approximately 20.2 debye is created from the movement of a single electronic charge over a distance of 4.2 Å during the passage from KSI–intermediate complex to KSI–substrate complex, which is similar in magnitude to  debye created during photoexcitation of enzyme-bound C183 (see text).
debye created during photoexcitation of enzyme-bound C183 (see text).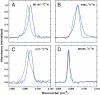
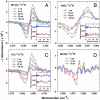
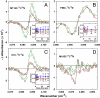
References
-
- Kraut J. How do enzymes work? Science. 1988;242:533–540. - PubMed
-
- Radzicka A, Wolfenden R. A proficient enzyme. Science. 1995;267:90–93. - PubMed
-
- Bruice TC. A view at the millennium: The efficiency of enzymatic catalysis. Acc Chem Res. 2002;35:139–148. - PubMed
-
- Kraut DA, Carroll KS, Herschlag D. Challenges in enzyme mechanism and energetics. Annu Rev Biochem. 2003;72:517–571. - PubMed
-
- Zhang X, Houk KN. Why enzymes are proficient catalysts: Beyond the Pauling paradigm. Acc Chem Res. 2005;38:379–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

