Inhibition recruitment in prefrontal cortex during sleep spindles and gating of hippocampal inputs
- PMID: 21949372
- PMCID: PMC3193185
- DOI: 10.1073/pnas.1103612108
Inhibition recruitment in prefrontal cortex during sleep spindles and gating of hippocampal inputs
Abstract
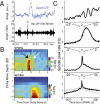
During light slow-wave sleep, the thalamo-cortical network oscillates in waxing-and-waning patterns at about 7 to 14 Hz and lasting for 500 ms to 3 s, called spindles, with the thalamus rhythmically sending strong excitatory volleys to the cortex. Concurrently, the hippocampal activity is characterized by transient and strong excitatory events, Sharp-Waves-Ripples (SPWRs), directly affecting neocortical activity--in particular the medial prefrontal cortex (mPFC)--which receives monosynaptic fibers from the ventral hippocampus and subiculum. Both spindles and SPWRs have been shown to be strongly involved in memory consolidation. However, the dynamics of the cortical network during natural sleep spindles and how prefrontal circuits simultaneously process hippocampal and thalamo-cortical activity remain largely undetermined. Using multisite neuronal recordings in rat mPFC, we show that during sleep spindles, oscillatory responses of cortical cells are different for different cell types and cortical layers. Superficial neurons are more phase-locked and tonically recruited during spindle episodes. Moreover, in a given layer, interneurons were always more modulated than pyramidal cells, both in firing rate and phase, suggesting that the dynamics are dominated by inhibition. In the deep layers, where most of the hippocampal fibers make contacts, pyramidal cells respond phasically to SPWRs, but not during spindles. Similar observations were obtained when analyzing γ-oscillation modulation in the mPFC. These results demonstrate that during sleep spindles, the cortex is functionnaly "deafferented" from its hippocampal inputs, based on processes of cortical origin, and presumably mediated by the strong recruitment of inhibitory interneurons. The interplay between hippocampal and thalamic inputs may underlie a global mechanism involved in the consolidation of recently formed memory traces.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Occurrence of Hippocampal Ripples is Associated with Activity Suppression in the Mediodorsal Thalamic Nucleus.J Neurosci. 2019 Jan 16;39(3):434-444. doi: 10.1523/JNEUROSCI.2107-18.2018. Epub 2018 Nov 20. J Neurosci. 2019. PMID: 30459228 Free PMC article.
-
Monosynaptic Hippocampal-Prefrontal Projections Contribute to Spatial Memory Consolidation in Mice.J Neurosci. 2019 Aug 28;39(35):6978-6991. doi: 10.1523/JNEUROSCI.2158-18.2019. Epub 2019 Jul 8. J Neurosci. 2019. PMID: 31285301 Free PMC article.
-
mPFC spindle cycles organize sparse thalamic activation and recently active CA1 cells during non-REM sleep.Elife. 2020 Jun 11;9:e48881. doi: 10.7554/eLife.48881. Elife. 2020. PMID: 32525480 Free PMC article.
-
Oscillations and hippocampal-prefrontal synchrony.Curr Opin Neurobiol. 2011 Jun;21(3):467-74. doi: 10.1016/j.conb.2011.04.006. Epub 2011 May 14. Curr Opin Neurobiol. 2011. PMID: 21571522 Free PMC article. Review.
-
Hippocampal ripples as a mode of communication with cortical and subcortical areas.Hippocampus. 2020 Jan;30(1):39-49. doi: 10.1002/hipo.22997. Epub 2018 Nov 13. Hippocampus. 2020. PMID: 30069976 Review.
Cited by
-
Posterior Hippocampal Spindle Ripples Co-occur with Neocortical Theta Bursts and Downstates-Upstates, and Phase-Lock with Parietal Spindles during NREM Sleep in Humans.J Neurosci. 2019 Nov 6;39(45):8949-8968. doi: 10.1523/JNEUROSCI.2858-18.2019. Epub 2019 Sep 17. J Neurosci. 2019. PMID: 31530646 Free PMC article.
-
Waveform detection by deep learning reveals multi-area spindles that are selectively modulated by memory load.Elife. 2022 Jun 29;11:e75769. doi: 10.7554/eLife.75769. Elife. 2022. PMID: 35766286 Free PMC article.
-
Responses in Rat Core Auditory Cortex are Preserved during Sleep Spindle Oscillations.Sleep. 2016 May 1;39(5):1069-82. doi: 10.5665/sleep.5758. Sleep. 2016. PMID: 26856904 Free PMC article.
-
Prefrontal cortical ripples mediate top-down suppression of hippocampal reactivation during sleep memory consolidation.Curr Biol. 2024 Jul 8;34(13):2801-2811.e9. doi: 10.1016/j.cub.2024.05.018. Epub 2024 Jun 3. Curr Biol. 2024. PMID: 38834064 Free PMC article.
-
Replay, the default mode network and the cascaded memory systems model.Nat Rev Neurosci. 2022 Oct;23(10):628-640. doi: 10.1038/s41583-022-00620-6. Epub 2022 Aug 15. Nat Rev Neurosci. 2022. PMID: 35970912 Review.
References
-
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. - PubMed
-
- Steriade M. Neuronal Substrates of Sleep and Epilepsy. Cambridge, United Kingdom: Cambridge Univ Press; 2003.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

