Control of Nanoscale Environment to Improve Stability of Immobilized Proteins on Diamond Surfaces
- PMID: 21949497
- PMCID: PMC3177702
- DOI: 10.1002/adfm.201002251
Control of Nanoscale Environment to Improve Stability of Immobilized Proteins on Diamond Surfaces
Abstract
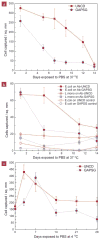
Immunoassays for detection of bacterial pathogens rely on the selectivity and stability of bio-recognition elements such as antibodies tethered to sensor surfaces. The search for novel surfaces that improve the stability of biomolecules and assay performance has been pursued for a long time. However, the anticipated improvements in stability have not been realized in practice under physiological conditions because the surface functionalization layers on commonly used substrates, silica and gold, are themselves unstable on time scales of days. In this paper, we show that covalent linking of antibodies to diamond surfaces leads to substantial improvements in biological activity of proteins as measured by the ability to selectively capture cells of the pathogenic bacterium Escherichia coli O157:H7 even after exposure to buffer solutions at 37 °C for extended periods of time, approaching 2 weeks. Our results from ELISA, XPS, fluorescence microscopy, and MD simulations suggest that by using highly stable surface chemistry and controlling the nanoscale organization of the antibodies on the surface, it is possible to achieve significant improvements in biological activity and stability. Our findings can be easily extended to functionalization of micro and nanodimensional sensors and structures of biomedical diagnostic and therapeutic interest.
Figures







Similar articles
-
Surface functionalization of thin-film diamond for highly stable and selective biological interfaces.Proc Natl Acad Sci U S A. 2011 Jan 18;108(3):983-8. doi: 10.1073/pnas.1006660107. Epub 2010 Sep 30. Proc Natl Acad Sci U S A. 2011. PMID: 20884854 Free PMC article.
-
Fiber optic surface plasmon resonance sensor for detection of E. coli O157:H7 based on antimicrobial peptides and AgNPs-rGO.Biosens Bioelectron. 2018 Oct 15;117:347-353. doi: 10.1016/j.bios.2018.06.005. Epub 2018 Jun 5. Biosens Bioelectron. 2018. PMID: 29935488
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
Design of surface modifications for nanoscale sensor applications.Sensors (Basel). 2015 Jan 14;15(1):1635-75. doi: 10.3390/s150101635. Sensors (Basel). 2015. PMID: 25594599 Free PMC article. Review.
-
Development of a low-cost paper-based ELISA method for rapid Escherichia coli O157:H7 detection.Anal Biochem. 2018 Feb 1;542:58-62. doi: 10.1016/j.ab.2017.11.010. Epub 2017 Nov 20. Anal Biochem. 2018. PMID: 29158131 Review.
Cited by
-
Microscopic Perspective on the Adsorption Isotherm of a Heterogeneous Surface.J Phys Chem Lett. 2011 Jul 2;2(14):1804-1807. doi: 10.1021/jz200749d. J Phys Chem Lett. 2011. PMID: 22611479 Free PMC article.
-
Atoms-to-microns model for small solute transport through sticky nanochannels.Lab Chip. 2011 Nov 21;11(22):3766-73. doi: 10.1039/c1lc20697d. Epub 2011 Oct 10. Lab Chip. 2011. PMID: 21986816 Free PMC article.
-
Nanostructuring of biosensing electrodes with nanodiamonds for antibody immobilization.ACS Nano. 2014 Feb 25;8(2):1419-28. doi: 10.1021/nn405240g. Epub 2014 Jan 10. ACS Nano. 2014. PMID: 24397797 Free PMC article.
-
In silico study of substrate chemistry effect on the tethering of engineered antibodies for SARS-CoV-2 detection: Amorphous silica vs gold.Colloids Surf B Biointerfaces. 2022 May;213:112400. doi: 10.1016/j.colsurfb.2022.112400. Epub 2022 Feb 7. Colloids Surf B Biointerfaces. 2022. PMID: 35158221 Free PMC article.
-
The effect of electrode size and surface heterogeneity on electrochemical properties of ultrananocrystalline diamond microelectrode.J Electroanal Chem (Lausanne). 2015 Nov 1;756:61-68. doi: 10.1016/j.jelechem.2015.08.016. Epub 2015 Aug 14. J Electroanal Chem (Lausanne). 2015. PMID: 32280318 Free PMC article.
References
-
- USDA/ERS. Foodborne Illness Cost Calculator. ( http://www.ers.usda.gov/Data/FoodBorneIllness/)
-
- Ivnitski D, Abdel-Hamid I, Atanasov P, Wilkins E. Biosensors Bio-electron. 1999;14:599. - PubMed
-
- Haynes CA, Norde W. Colloids Surfaces B. 1994;2:517.
-
- Jeon SI, Lee JH, Andrade JD, Degennes PG. J Colloid Interface Sci. 1991;142:149.
-
- Giacomelli CE, Bremer MGEG, Norde W. J Colloid Interface Sci. 1999;220:13. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
