Galectin-1-mediated biochemical controls of melanoma and glioma aggressive behavior
- PMID: 21949569
- PMCID: PMC3178756
- DOI: 10.4331/wjbc.v2.i9.193
Galectin-1-mediated biochemical controls of melanoma and glioma aggressive behavior
Abstract
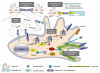
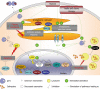
Gliomas and melanomas are associated with dismal prognosis because of their marked intrinsic resistance to proapoptotic stimuli, such as conventional chemotherapy and radiotherapy, as well as their ability to escape immune cell attacks. In addition, gliomas and melanomas display pronounced neoangiogenesis. Galectin-1 is a hypoxia-sensitive protein, which is abundantly secreted by glioma and melanoma cells, which displays marked proangiogenic effects. It also provides immune tolerogenic environments to melanoma and glioma cells through the killing of activated T cells that attack these tumor cells. Galectin-1 protects glioma and melanoma cells against cytotoxic insults (including chemotherapy and radiotherapy) through a direct role in the unfolded protein response. Altogether, these facts clearly point to galectin-1 as an important target to be combated in gliomas and melanomas in order to: (1) weaken the defenses of these two types of cancers against radiotherapy, chemotherapy and immunotherapy/vaccine therapy; and (2) reinforce antiangiogenic therapies. In the present article, we review the biochemical and molecular biology-related pathways controlled by galectin-1, which are actually beneficial for melanoma and glioma cells, and therefore detrimental for melanoma and glioma patients.
Keywords: Biochemical pathways; Galectin-1; Glioma; Melanoma.
Figures


References
-
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. - PubMed
-
- Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim Biophys Acta. 2002;1572:285–293. - PubMed
-
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. - PubMed
-
- Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. - PubMed
-
- Houzelstein D, Gonçalves IR, Fadden AJ, Sidhu SS, Cooper DN, Drickamer K, Leffler H, Poirier F. Phylogenetic analysis of the vertebrate galectin family. Mol Biol Evol. 2004;21:1177–1187. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

