The Effect of Synovial Fluid Enzymes on the Biodegradability of Collagen and Fibrin Clots
- PMID: 21949586
- PMCID: PMC3176731
- DOI: 10.3390/ma4081469
The Effect of Synovial Fluid Enzymes on the Biodegradability of Collagen and Fibrin Clots
Abstract
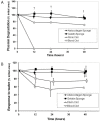
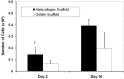
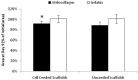
Recently there has been a great deal of interest in the use of biomaterials to stimulate wound healing. This is largely due to their ability to centralize high concentrations of compounds known to promote wound healing at a needed location. Joints present a unique challenge to using scaffolds because of the presence of enzymes in synovial fluid which are known to degrade materials that would be stable in other parts of the body. The hypothesis of this study was that atelocollagen scaffolds would have greater resistance to enzymatic degradation than scaffolds made of gelatin, fibrin and whole blood. To test this hypothesis, collagen and fibrin-based scaffolds were placed in matrix metallopeptidase-1 (MMP-1), elastase, and plasmin solutions at physiologic concentrations, and the degradation of each scaffold was measured at varying time points. The atelocollagen scaffolds had a significantly greater resistance to degradation by MMP-1, elastase and plasmin over the fibrin based scaffolds. The results suggest that atelocollagen-based scaffolds may provide some protection against premature degradation by synovial fluid enzymes over fibrin-based matrices.
Figures


Similar articles
-
Noninvasive Fibrin Targeting Colloid-Mediated Intra-Articular Repair.J Biomed Mater Res A. 2025 Apr;113(4):e37901. doi: 10.1002/jbm.a.37901. J Biomed Mater Res A. 2025. PMID: 40145327
-
Fibrin degradation in the synovial fluid of rheumatoid arthritis patients: a model for extravascular fibrinolysis.Semin Thromb Hemost. 1996;22(6):489-96. doi: 10.1055/s-2007-999049. Semin Thromb Hemost. 1996. PMID: 9122713 Review.
-
Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering.Acta Biomater. 2016 Oct 1;43:122-138. doi: 10.1016/j.actbio.2016.07.043. Epub 2016 Jul 27. Acta Biomater. 2016. PMID: 27475528 Free PMC article.
-
Evaluation of bone matrix gelatin/fibrin glue and chitosan/gelatin composite scaffolds for cartilage tissue engineering.Genet Mol Res. 2016 Jul 15;15(3). doi: 10.4238/gmr.15038431. Genet Mol Res. 2016. PMID: 27525846
-
Modified fibrin hydrogel matrices: both, 3D-scaffolds and local and controlled release systems to stimulate angiogenesis.Curr Pharm Des. 2007;13(35):3597-607. doi: 10.2174/138161207782794158. Curr Pharm Des. 2007. PMID: 18220797 Review.
Cited by
-
The importance of plasmin for the healing of the anterior cruciate ligament.Bone Joint Res. 2020 Sep 3;9(9):543-553. doi: 10.1302/2046-3758.99.BJR-2020-0048.R1. eCollection 2020 Sep. Bone Joint Res. 2020. PMID: 32922763 Free PMC article.
-
Multiple-Factors-Induced Rheumatoid Arthritis Synoviocyte Activation Is Attenuated by the α2-Adrenergic Receptor Agonist Dexmedetomidine.Int J Mol Sci. 2023 Jun 28;24(13):10756. doi: 10.3390/ijms241310756. Int J Mol Sci. 2023. PMID: 37445932 Free PMC article.
-
Bench-to-bedside: Bridge-enhanced anterior cruciate ligament repair.J Orthop Res. 2017 Dec;35(12):2606-2612. doi: 10.1002/jor.23632. Epub 2017 Jul 9. J Orthop Res. 2017. PMID: 28608618 Free PMC article. Review.
-
Fibrin-Based Biomaterial Systems to Enhance Anterior Cruciate Ligament Healing.Med Devices Sens. 2021 Feb;4(1):e10147. doi: 10.1002/mds3.10147. Epub 2020 Nov 11. Med Devices Sens. 2021. PMID: 34458685 Free PMC article.
-
Suture Anchor Technique for Bridge Enhanced Anterior Cruciate Ligament Restoration.Arthrosc Tech. 2024 Jan 1;13(3):102880. doi: 10.1016/j.eats.2023.11.008. eCollection 2024 Mar. Arthrosc Tech. 2024. PMID: 38584620 Free PMC article.
References
-
- Murray M.M., Spindler K.P., Abreu E., Muller J.A., Nedder A., Kelly M., Frino J., Zurakowski D., Valenza M., Snyder B.D., et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J. Orthop. Res. 2007;25:81–91. doi: 10.1002/jor.20282. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
