Erythrocyte and porcine intestinal glycosphingolipids recognized by F4 fimbriae of enterotoxigenic Escherichia coli
- PMID: 21949679
- PMCID: PMC3174951
- DOI: 10.1371/journal.pone.0023309
Erythrocyte and porcine intestinal glycosphingolipids recognized by F4 fimbriae of enterotoxigenic Escherichia coli
Abstract
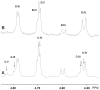
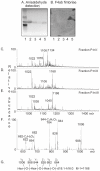
Enterotoxigenic F4-fimbriated Escherichia coli is associated with diarrheal disease in neonatal and postweaning pigs. The F4 fimbriae mediate attachment of the bacteria to the pig intestinal epithelium, enabling an efficient delivery of diarrhea-inducing enterotoxins to the target epithelial cells. There are three variants of F4 fimbriae designated F4ab, F4ac and F4ad, respectively, having different antigenic and adhesive properties. In the present study, the binding of isolated F4ab, F4ac and F4ad fimbriae, and F4ab/ac/ad-fimbriated E. coli, to glycosphingolipids from erythrocytes and from porcine small intestinal epithelium was examined, in order to get a comprehensive view of the F4-binding glycosphingolipids involved in F4-mediated hemagglutination and adhesion to the epithelial cells of porcine intestine. Specific interactions between the F4ab, F4ac and F4ad fimbriae and both acid and non-acid glycosphingolipids were obtained, and after isolation of binding-active glycosphingolipids and characterization by mass spectrometry and proton NMR, distinct carbohydrate binding patterns were defined for each fimbrial subtype. Two novel glycosphingolipids were isolated from chicken erythrocytes, and characterized as GalNAcα3GalNAcß3Galß4Glcß1Cer and GalNAcα3GalNAcß3Galß4GlcNAcß3Galß4Glcß1Cer. These two compounds, and lactosylceramide (Galß4Glcß1Cer) with phytosphingosine and hydroxy fatty acid, were recognized by all three variants of F4 fimbriae. No binding of the F4ad fimbriae or F4ad-fimbriated E. coli to the porcine intestinal glycosphingolipids occurred. However, for F4ab and F4ac two distinct binding patterns were observed. The F4ac fimbriae and the F4ac-expressing E. coli selectively bound to galactosylceramide (Galß1Cer) with sphingosine and hydroxy 24:0 fatty acid, while the porcine intestinal glycosphingolipids recognized by F4ab fimbriae and the F4ab-fimbriated bacteria were characterized as galactosylceramide, sulfatide (SO(3)-3Galß1Cer), sulf-lactosylceramide (SO(3)-3Galß4Glcß1Cer), and globotriaosylceramide (Galα4Galß4Glcß1Cer) with phytosphingosine and hydroxy 24:0 fatty acid. Finally, the F4ad fimbriae and the F4ad-fimbriated E. coli, but not the F4ab or F4ac subtypes, bound to reference gangliotriaosylceramide (GalNAcß4Galß4Glcß1Cer), gangliotetraosylceramide (Galß3GalNAcß4Galß4Glcß1Cer), isoglobotriaosylceramide (Galα3Galß4Glcß1Cer), and neolactotetraosylceramide (Galß4GlcNAcß3Galß4Glcß1Cer).
Conflict of interest statement
Figures










References
-
- Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Re. 2005;6:17–39. - PubMed
-
- Van den Broeck W, Cox E, Oudega B, Goddeeris BM. The F4 fimbrial antigen of Escherichia coli and its receptors. Vet Microbiol. 2000;71:223–244. - PubMed
-
- Mol O, Oudega B. Molecular and structural aspects of fimbriae biosynthesis and assembly in Escherichia coli. FEMS Microbiol Rev. 1996;19:25–52. - PubMed
-
- Oudega B, de Graaf M, de Boer L, Bakker D, Vader CE, et al. Detection and identification of FaeC as a minor component of K88 fibrillae of Escherichia coli. Mol Microbiol. 1989;3:645–652. - PubMed
-
- Valent QA, Zaal J, de Graaf FK, Oudega B. Subcellular localization and topology of the K88 usher FaeD in Escherichia coli. Mol Microbiol. 1995;16:1243–1257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

