PS integrins and laminins: key regulators of cell migration during Drosophila embryogenesis
- PMID: 21949686
- PMCID: PMC3174947
- DOI: 10.1371/journal.pone.0023893
PS integrins and laminins: key regulators of cell migration during Drosophila embryogenesis
Abstract
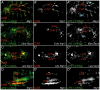
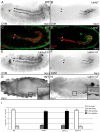
During embryonic development, there are numerous cases where organ or tissue formation depends upon the migration of primordial cells. In the Drosophila embryo, the visceral mesoderm (vm) acts as a substrate for the migration of several cell populations of epithelial origin, including the endoderm, the trachea and the salivary glands. These migratory processes require both integrins and laminins. The current model is that αPS1βPS (PS1) and/or αPS3βPS (PS3) integrins are required in migrating cells, whereas αPS2βPS (PS2) integrin is required in the vm, where it performs an as yet unidentified function. Here, we show that PS1 integrins are also required for the migration over the vm of cells of mesodermal origin, the caudal visceral mesoderm (CVM). These results support a model in which PS1 might have evolved to acquire the migratory function of integrins, irrespective of the origin of the tissue. This integrin function is highly specific and its specificity resides mainly in the extracellular domain. In addition, we have identified the Laminin α1,2 trimer, as the key extracellular matrix (ECM) component regulating CVM migration. Furthermore, we show that, as it is the case in vertebrates, integrins, and specifically PS2, contributes to CVM movement by participating in the correct assembly of the ECM that serves as tracks for migration.
Conflict of interest statement
Figures






References
-
- Bokel C, Brown NH. Integrins in Development: moving on, respoding to, and sticking to the extracellular matrix. Developmental Cell. 2002;3:311–321. - PubMed
-
- Roote CE, Zusman S. Functions for PS integrins in tissue adhesion, migration, and shape changes during early embryonic development in Drosophila. Dev Biol. 1995;169:322–336. - PubMed
-
- Martin-Bermudo MD, Alvarez-Garcia I, Brown NH. Migration of the drosophila primordial midgut cells requires coordination of diverse PS integrin functions. Development. 1999;126:5161–5169. - PubMed
-
- Devenport D, Brown NH. Morphogenesis in the absence of integrins:mutation of both Drosophila βsubunits prevents midgut migration. Development. 2004;131:5405–5415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

