Pathogenic Neisseria hitchhike on the uropod of human neutrophils
- PMID: 21949708
- PMCID: PMC3174955
- DOI: 10.1371/journal.pone.0024353
Pathogenic Neisseria hitchhike on the uropod of human neutrophils
Abstract
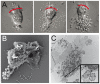
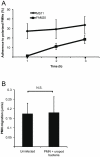
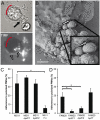
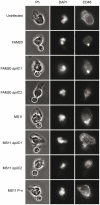
Polymorphonuclear neutrophils (PMNs) are important components of the human innate immune system and are rapidly recruited at the site of bacterial infection. Despite the effective phagocytic activity of PMNs, Neisseria gonorrhoeae infections are characterized by high survival within PMNs. We reveal a novel type IV pilus-mediated adherence of pathogenic Neisseria to the uropod (the rear) of polarized PMNs. The direct pilus-uropod interaction was visualized by scanning electron microscopy and total internal reflection fluorescence (TIRF) microscopy. We showed that N. meningitidis adhesion to the PMN uropod depended on both pilus-associated proteins PilC1 and PilC2, while N. gonorrhoeae adhesion did not. Bacterial adhesion elicited accumulation of the complement regulator CD46, but not I-domain-containing integrins, beneath the adherent bacterial microcolony. Electrographs and live-cell imaging of PMNs suggested that bacterial adherence to the uropod is followed by internalization into PMNs via the uropod. We also present data showing that pathogenic Neisseria can hitchhike on PMNs to hide from their phagocytic activity as well as to facilitate the spread of the pathogen through the epithelial cell layer.
Conflict of interest statement
Figures

Similar articles
-
PilC of Neisseria meningitidis is involved in class II pilus formation and restores pilus assembly, natural transformation competence and adherence to epithelial cells in PilC-deficient gonococci.Mol Microbiol. 1997 Mar;23(5):879-92. doi: 10.1046/j.1365-2958.1997.2631630.x. Mol Microbiol. 1997. PMID: 9076726
-
Mutation of the conserved calcium-binding motif in Neisseria gonorrhoeae PilC1 impacts adhesion but not piliation.Infect Immun. 2013 Nov;81(11):4280-9. doi: 10.1128/IAI.00493-13. Epub 2013 Sep 3. Infect Immun. 2013. PMID: 24002068 Free PMC article.
-
Neisseria PilC protein identified as type-4 pilus tip-located adhesin.Nature. 1995 Jan 26;373(6512):357-9. doi: 10.1038/373357a0. Nature. 1995. PMID: 7830772
-
Interaction of pathogenic neisseriae with nonphagocytic cells.Clin Microbiol Rev. 1995 Jul;8(3):376-88. doi: 10.1128/CMR.8.3.376. Clin Microbiol Rev. 1995. PMID: 7553571 Free PMC article. Review.
-
Interactions of pathogenic neisseriae with epithelial cell membranes.Annu Rev Cell Dev Biol. 2000;16:423-57. doi: 10.1146/annurev.cellbio.16.1.423. Annu Rev Cell Dev Biol. 2000. PMID: 11031243 Review.
Cited by
-
A bacterial siren song: intimate interactions between Neisseria and neutrophils.Nat Rev Microbiol. 2012 Jan 31;10(3):178-90. doi: 10.1038/nrmicro2713. Nat Rev Microbiol. 2012. PMID: 22290508 Free PMC article. Review.
-
Immune responses to Neisseria gonorrhoeae and implications for vaccine development.Front Immunol. 2023 Aug 17;14:1248613. doi: 10.3389/fimmu.2023.1248613. eCollection 2023. Front Immunol. 2023. PMID: 37662926 Free PMC article. Review.
-
Triple co-culture and perfusion bioreactor for studying the interaction between Neisseria gonorrhoeae and neutrophils: A novel 3D tissue model for bacterial infection and immunity.J Tissue Eng. 2021 Jan 28;12:2041731420988802. doi: 10.1177/2041731420988802. eCollection 2021 Jan-Dec. J Tissue Eng. 2021. PMID: 33796248 Free PMC article.
-
The role of pili in Bacillus cereus intraocular infection.Exp Eye Res. 2017 Jun;159:69-76. doi: 10.1016/j.exer.2017.03.007. Epub 2017 Mar 20. Exp Eye Res. 2017. PMID: 28336259 Free PMC article.
-
Neisseria gonorrhoeae subverts formin-dependent actin polymerization to colonize human macrophages.PLoS Pathog. 2021 Dec 28;17(12):e1010184. doi: 10.1371/journal.ppat.1010184. eCollection 2021 Dec. PLoS Pathog. 2021. PMID: 34962968 Free PMC article.
References
-
- Coureuil M, Lecuyer H, Scott MG, Boularan C, Enslen H, et al. Meningococcus Hijacks a beta2-adrenoceptor/beta-Arrestin pathway to cross brain microvasculature endothelium. Cell. 2010;143:1149–1160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

