A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide® ISA 720
- PMID: 21949716
- PMCID: PMC3176224
- DOI: 10.1371/journal.pone.0024413
A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide® ISA 720
Abstract
Background: In a previous Phase 1/2b malaria vaccine trial testing the 3D7 isoform of the malaria vaccine candidate Merozoite surface protein 2 (MSP2), parasite densities in children were reduced by 62%. However, breakthrough parasitemias were disproportionately of the alternate dimorphic form of MSP2, the FC27 genotype. We therefore undertook a dose-escalating, double-blinded, placebo-controlled Phase 1 trial in healthy, malaria-naïve adults of MSP2-C1, a vaccine containing recombinant forms of the two families of msp2 alleles, 3D7 and FC27 (EcMSP2-3D7 and EcMSP2-FC27), formulated in equal amounts with Montanide® ISA 720 as a water-in-oil emulsion.
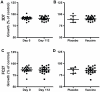
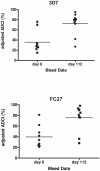
Methodology/principal findings: The trial was designed to include three dose cohorts (10, 40, and 80 µg), each with twelve subjects receiving the vaccine and three control subjects receiving Montanide® ISA 720 adjuvant emulsion alone, in a schedule of three doses at 12-week intervals. Due to unexpected local reactogenicity and concern regarding vaccine stability, the trial was terminated after the second immunisation of the cohort receiving the 40 µg dose; no subjects received the 80 µg dose. Immunization induced significant IgG responses to both isoforms of MSP2 in the 10 µg and 40 µg dose cohorts, with antibody levels by ELISA higher in the 40 µg cohort. Vaccine-induced antibodies recognised native protein by Western blots of parasite protein extracts and by immunofluorescence microscopy. Although the induced anti-MSP2 antibodies did not directly inhibit parasite growth in vitro, IgG from the majority of individuals tested caused significant antibody-dependent cellular inhibition (ADCI) of parasite growth.
Conclusions/significance: As the majority of subjects vaccinated with MSP2-C1 developed an antibody responses to both forms of MSP2, and that these antibodies mediated ADCI provide further support for MSP2 as a malaria vaccine candidate. However, in view of the reactogenicity of this formulation, further clinical development of MSP2-C1 will require formulation of MSP2 in an alternative adjuvant.
Trial registration: Australian New Zealand Clinical Trials Registry 12607000552482.
Conflict of interest statement
Figures
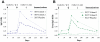

References
-
- WHO. 2009. World Malaria Report.
-
- Anders RF, Adda CG, Foley M, Norton RS. Recombinant protein vaccines against the asexual blood stages of Plasmodium falciparum. Hum Vaccin. 2010;6:39–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

