Structure and molecular evolution of CDGSH iron-sulfur domains
- PMID: 21949752
- PMCID: PMC3174974
- DOI: 10.1371/journal.pone.0024790
Structure and molecular evolution of CDGSH iron-sulfur domains
Abstract
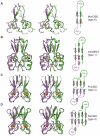
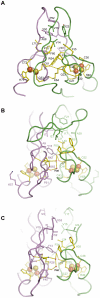
The recently discovered CDGSH iron-sulfur domains (CISDs) are classified into seven major types with a wide distribution throughout the three domains of life. The type 1 protein mitoNEET has been shown to fold into a dimer with the signature CDGSH motif binding to a [2Fe-2S] cluster. However, the structures of all other types of CISDs were unknown. Here we report the crystal structures of type 3, 4, and 6 CISDs determined at 1.5 Å, 1.8 Å and 1.15 Å resolution, respectively. The type 3 and 4 CISD each contain one CDGSH motif and adopt a dimeric structure. Although similar to each other, the two structures have permutated topologies, and both are distinct from the type 1 structure. The type 6 CISD contains tandem CDGSH motifs and adopts a monomeric structure with an internal pseudo dyad symmetry. All currently known CISD structures share dual iron-sulfur binding modules and a β-sandwich for either intermolecular or intramolecular dimerization. The iron-sulfur binding module, the β-strand N-terminal to the module and a proline motif are conserved among different type structures, but the dimerization module and the interface and orientation between the two iron-sulfur binding modules are divergent. Sequence analysis further shows resemblance between CISD types 4 and 7 and between 1 and 2. Our findings suggest that all CISDs share common ancestry and diverged into three primary folds with a characteristic phylogenetic distribution: a eukaryote-specific fold adopted by types 1 and 2 proteins, a prokaryote-specific fold adopted by types 3, 4 and 7 proteins, and a tandem-motif fold adopted by types 5 and 6 proteins. Our comprehensive structural, sequential and phylogenetic analysis provides significant insight into the assembly principles and evolutionary relationship of CISDs.
Conflict of interest statement
Figures




References
-
- Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. - PubMed
-
- Beinert H. Iron-sulfur proteins: ancient structures, still full of surprises. J Biol Inorg Chem. 2000;5:2–15. - PubMed
-
- Beinert H, Holm RH, Munck E. Iron-sulfur clusters: nature's modular, multipurpose structures. Science. 1997;277:653–659. - PubMed
-
- Wiley SE, Paddock ML, Abresch EC, Gross L, van der Geer P, et al. The outer mitochondrial membrane protein mitoNEET contains a novel redox-active 2Fe-2S cluster. J Biol Chem. 2007;282:23745–23749. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

