Simultaneous inhibition of mTOR-containing complex 1 (mTORC1) and MNK induces apoptosis of cutaneous T-cell lymphoma (CTCL) cells
- PMID: 21949767
- PMCID: PMC3174990
- DOI: 10.1371/journal.pone.0024849
Simultaneous inhibition of mTOR-containing complex 1 (mTORC1) and MNK induces apoptosis of cutaneous T-cell lymphoma (CTCL) cells
Retraction in
-
Retraction: Simultaneous Inhibition of mTOR-Containing Complex 1 (mTORC1) and MNK Induces Apoptosis of Cutaneous T-Cell Lymphoma (CTCL) Cells.PLoS One. 2020 Sep 10;15(9):e0239102. doi: 10.1371/journal.pone.0239102. eCollection 2020. PLoS One. 2020. PMID: 32913370 Free PMC article. No abstract available.
Abstract
Background: mTOR kinase forms the mTORC1 complex by associating with raptor and other proteins and affects a number of key cell functions. mTORC1 activates p70S6kinase 1 (p70S6K1) and inhibits 4E-binding protein 1 (4E-BP1). In turn, p70S6K1 phosphorylates a S6 protein of the 40S ribosomal subunit (S6rp) and 4E-BP1, with the latter negatively regulating eukaryotic initiation factor 4E (eIF-4E). MNK1 and MNK2 kinases phosphorylate and augment activity of eIF4E. Rapamycin and its analogs are highly specific, potent, and relatively non-toxic inhibitors of mTORC1. Although mTORC1 activation is present in many types of malignancies, rapamycin-type inhibitors shows relatively limited clinical efficacy as single agents. Initially usually indolent, CTCL displays a tendency to progress to the aggressive forms with limited response to therapy and poor prognosis. Our previous study (M. Marzec et al. 2008) has demonstrated that CTCL cells display mTORC1 activation and short-term treatment of CTCL-derived cells with rapamycin suppressed their proliferation and had little effect on the cell survival.
Methods: Cells derived from CTCL were treated with mTORC1 inhibitor rapamycin and MNK inhibitor and evaluated for inhibition of the mTORC1 signaling pathway and cell growth and survival.
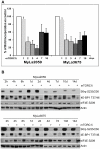
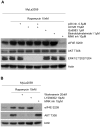
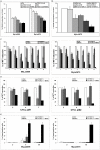
Results: Whereas the treatment with rapamycin persistently inhibited mTORC1 signaling, it suppressed only partially the cell growth. MNK kinase mediated the eIF4E phosphorylation and inhibition or depletion of MNK markedly suppressed proliferation of the CTCL cells when combined with the rapamycin-mediated inhibition of mTORC1. While MNK inhibition alone mildly suppressed the CTCL cell growth, the combined MNK and mTORC1 inhibition totally abrogated the growth. Similarly, MNK inhibitor alone displayed a minimal pro-apoptotic effect; in combination with rapamycin it triggered profound cell apoptosis.
Conclusions: These findings indicate that the combined inhibition of mTORC1 and MNK may prove beneficial in the treatment of CTCL and other malignancies.
Conflict of interest statement
Figures

Similar articles
-
Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3-kinase-dependent and Mnk-mediated eukaryotic translation initiation factor 4E phosphorylation.Mol Cell Biol. 2007 Nov;27(21):7405-13. doi: 10.1128/MCB.00760-07. Epub 2007 Aug 27. Mol Cell Biol. 2007. PMID: 17724079 Free PMC article.
-
CGP57380 enhances efficacy of RAD001 in non-small cell lung cancer through abrogating mTOR inhibition-induced phosphorylation of eIF4E and activating mitochondrial apoptotic pathway.Oncotarget. 2016 May 10;7(19):27787-801. doi: 10.18632/oncotarget.8497. Oncotarget. 2016. PMID: 27050281 Free PMC article.
-
Regulatory effects of a Mnk2-eIF4E feedback loop during mTORC1 targeting of human medulloblastoma cells.Oncotarget. 2014 Sep 30;5(18):8442-51. doi: 10.18632/oncotarget.2319. Oncotarget. 2014. PMID: 25193863 Free PMC article.
-
Targeting Mnks for cancer therapy.Oncotarget. 2012 Feb;3(2):118-31. doi: 10.18632/oncotarget.453. Oncotarget. 2012. PMID: 22392765 Free PMC article. Review.
-
Lost in translation: a neglected mTOR target for lymphangioleiomyomatosis.Eur Respir Rev. 2023 Sep 27;32(169):230100. doi: 10.1183/16000617.0100-2023. Print 2023 Sep 30. Eur Respir Rev. 2023. PMID: 37758276 Free PMC article. Review.
Cited by
-
Phosphorylation of the mRNA cap-binding protein eIF4E and cancer.Cell Signal. 2020 Sep;73:109689. doi: 10.1016/j.cellsig.2020.109689. Epub 2020 Jun 11. Cell Signal. 2020. PMID: 32535199 Free PMC article. Review.
-
Reciprocal signaling between mTORC1 and MNK2 controls cell growth and oncogenesis.Cell Mol Life Sci. 2021 Jan;78(1):249-270. doi: 10.1007/s00018-020-03491-1. Epub 2020 Mar 13. Cell Mol Life Sci. 2021. PMID: 32170339 Free PMC article.
-
The MNK1/2-eIF4E Axis as a Potential Therapeutic Target in Melanoma.Int J Mol Sci. 2020 Jun 5;21(11):4055. doi: 10.3390/ijms21114055. Int J Mol Sci. 2020. PMID: 32517051 Free PMC article. Review.
-
Inhibition of Mitogen-activated Protein Kinase (MAPK)-interacting Kinase (MNK) Preferentially Affects Translation of mRNAs Containing Both a 5'-Terminal Cap and Hairpin.J Biol Chem. 2016 Feb 12;291(7):3455-67. doi: 10.1074/jbc.M115.694190. Epub 2015 Dec 14. J Biol Chem. 2016. PMID: 26668315 Free PMC article.
-
Rapamycin Suppresses Tumor Growth and Alters the Metabolic Phenotype in T-Cell Lymphoma.J Invest Dermatol. 2015 Sep;135(9):2301-2308. doi: 10.1038/jid.2015.153. Epub 2015 Apr 21. J Invest Dermatol. 2015. PMID: 25897830
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

