The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation
- PMID: 21949865
- PMCID: PMC3176312
- DOI: 10.1371/journal.pone.0025109
The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation
Abstract
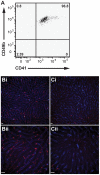
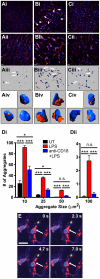
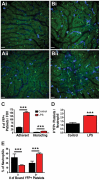
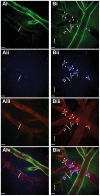
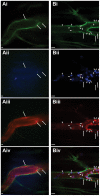
Platelets are central players in inflammation and are an important component of the innate immune response. The ability to visualize platelets within the live host is essential to understanding their role in these processes. Past approaches have involved adoptive transfer of labelled platelets, non-specific dyes, or the use of fluorescent antibodies to tag platelets in vivo. Often, these techniques result in either the activation of the platelet, or blockade of specific platelet receptors. In this report, we describe two new methods for intravital visualization of platelet biology, intravenous administration of labelled anti-CD49b, which labels all platelets, and CD41-YFP transgenic mice, in which a percentage of platelets express YFP. Both approaches label endogenous platelets and allow for their visualization using spinning-disk confocal fluorescent microscopy. Following LPS-induced inflammation, we were able to measure a significant increase in both the number and size of platelet aggregates observed within the vasculature of a number of different tissues. Real-time observation of these platelet aggregates reveals them to be large, dynamic structures that are continually expanding and sloughing-off into circulation. Using these techniques, we describe for the first time, platelet recruitment to, and behaviour within numerous tissues of the mouse, both under control conditions and following LPS induced inflammation.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

