A single amino acid mutation in SNAP-25 induces anxiety-related behavior in mouse
- PMID: 21949876
- PMCID: PMC3176821
- DOI: 10.1371/journal.pone.0025158
A single amino acid mutation in SNAP-25 induces anxiety-related behavior in mouse
Abstract
Synaptosomal-associated protein of 25 kDa (SNAP-25) is a presynaptic protein essential for neurotransmitter release. Previously, we demonstrate that protein kinase C (PKC) phosphorylates Ser(187) of SNAP-25, and enhances neurotransmitter release by recruiting secretory vesicles near to the plasma membrane. As PKC is abundant in the brain and SNAP-25 is essential for synaptic transmission, SNAP-25 phosphorylation is likely to play a crucial role in the central nervous system. We therefore generated a mutant mouse, substituting Ser(187) of SNAP-25 with Ala using "knock-in" technology. The most striking effect of the mutation was observed in their behavior. The homozygous mutant mice froze readily in response to environmental change, and showed strong anxiety-related behavior in general activity and light and dark preference tests. In addition, the mutant mice sometimes exhibited spontaneously occurring convulsive seizures. Microdialysis measurements revealed that serotonin and dopamine release were markedly reduced in amygdala. These results clearly indicate that PKC-dependent SNAP-25 phosphorylation plays a critical role in the regulation of emotional behavior as well as the suppression of epileptic seizures, and the lack of enhancement of monoamine release is one of the possible mechanisms underlying these defects.
Conflict of interest statement
Figures

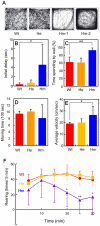
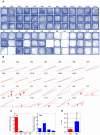
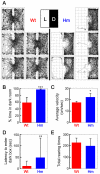

Similar articles
-
Protein kinase C-mediated translocation of secretory vesicles to plasma membrane and enhancement of neurotransmitter release from PC12 cells.Eur J Neurosci. 2002 Apr;15(8):1390-4. doi: 10.1046/j.1460-9568.2002.01972.x. Eur J Neurosci. 2002. PMID: 11994133
-
Epileptogenesis and epileptic maturation in phosphorylation site-specific SNAP-25 mutant mice.Epilepsy Res. 2015 Sep;115:30-44. doi: 10.1016/j.eplepsyres.2015.05.004. Epub 2015 May 19. Epilepsy Res. 2015. PMID: 26220374
-
SNAP-25 phosphorylation at Ser187 is not involved in Ca2+ or phorbolester-dependent potentiation of synaptic release.Mol Cell Neurosci. 2020 Jan;102:103452. doi: 10.1016/j.mcn.2019.103452. Epub 2019 Nov 30. Mol Cell Neurosci. 2020. PMID: 31794878
-
Inhibition of SNAP-25 phosphorylation at Ser187 is involved in chronic morphine-induced down-regulation of SNARE complex formation.J Biol Chem. 2004 Sep 24;279(39):40601-8. doi: 10.1074/jbc.M406896200. Epub 2004 Jul 23. J Biol Chem. 2004. PMID: 15277518
-
nPKCε Mediates SNAP-25 Phosphorylation of Ser-187 in Basal Conditions and After Synaptic Activity at the Neuromuscular Junction.Mol Neurobiol. 2019 Aug;56(8):5346-5364. doi: 10.1007/s12035-018-1462-5. Epub 2019 Jan 3. Mol Neurobiol. 2019. PMID: 30607888
Cited by
-
Accumulation of α-synuclein triggered by presynaptic dysfunction.J Neurosci. 2012 Nov 28;32(48):17186-96. doi: 10.1523/JNEUROSCI.2220-12.2012. J Neurosci. 2012. PMID: 23197711 Free PMC article.
-
SNAP-25, a Known Presynaptic Protein with Emerging Postsynaptic Functions.Front Synaptic Neurosci. 2016 Mar 24;8:7. doi: 10.3389/fnsyn.2016.00007. eCollection 2016. Front Synaptic Neurosci. 2016. PMID: 27047369 Free PMC article. Review.
-
Room for Two: The Synaptophysin/Synaptobrevin Complex.Front Synaptic Neurosci. 2021 Sep 20;13:740318. doi: 10.3389/fnsyn.2021.740318. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34616284 Free PMC article.
-
Protein instability, haploinsufficiency, and cortical hyper-excitability underlie STXBP1 encephalopathy.Brain. 2018 May 1;141(5):1350-1374. doi: 10.1093/brain/awy046. Brain. 2018. PMID: 29538625 Free PMC article.
-
SNAP-25 phosphorylation at Ser187 regulates synaptic facilitation and short-term plasticity in an age-dependent manner.Sci Rep. 2017 Aug 11;7(1):7996. doi: 10.1038/s41598-017-08237-x. Sci Rep. 2017. PMID: 28801590 Free PMC article.
References
-
- Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. - PubMed
-
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
-
- Hong W. SNAREs and traffic. Biochim Biophys Acta. 2005;1744:120–144. - PubMed
-
- Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. - PubMed
-
- Turner KM, Burgoyne RD, Morgan A. Protein phosphorylation and the regulation of synaptic membrane traffic. Trends Neurosci. 1999;22:459–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

