Targeting pannexin1 improves seizure outcome
- PMID: 21949881
- PMCID: PMC3175002
- DOI: 10.1371/journal.pone.0025178
Targeting pannexin1 improves seizure outcome
Abstract
Imbalance of the excitatory neurotransmitter glutamate and of the inhibitory neurotransmitter GABA is one of several causes of seizures. ATP has also been implicated in epilepsy. However, little is known about the mechanisms involved in the release of ATP from cells and the consequences of the altered ATP signaling during seizures. Pannexin1 (Panx1) is found in astrocytes and in neurons at high levels in the embryonic and young postnatal brain, declining in adulthood. Panx1 forms large-conductance voltage sensitive plasma membrane channels permeable to ATP that are also activated by elevated extracellular K(+) and following P2 receptor stimulation. Based on these properties, we hypothesized that Panx1 channels may contribute to seizures by increasing the levels of extracellular ATP. Using pharmacological tools and two transgenic mice deficient for Panx1 we show here that interference with Panx1 ameliorates the outcome and shortens the duration of kainic acid-induced status epilepticus. These data thus indicate that the activation of Panx1 in juvenile mouse hippocampi contributes to neuronal hyperactivity in seizures.
Conflict of interest statement
Figures
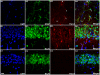
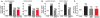
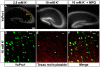

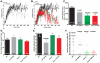
References
-
- Wieraszko A, Seyfried TN. Increased amount of extracellular ATP in stimulated hippocampal slices of seizure prone mice. Neurosci Lett. 1989;106:287–293. - PubMed
-
- Lopatar J, Dale N, Frenguelli BG. Minor contribution of ATP P2 receptors to electrically-evoked electrographic seizure activity in hippocampal slices: Evidence from purine biosensors and P2 receptor agonists and antagonists. Neuropharmacology. 2011;61:25–34. - PubMed
-
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. - PubMed
-
- Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, et al. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

