The structure and function of the glucagon-like peptide-1 receptor and its ligands
- PMID: 21950636
- PMCID: PMC3415635
- DOI: 10.1111/j.1476-5381.2011.01687.x
The structure and function of the glucagon-like peptide-1 receptor and its ligands
Abstract
Glucagon-like peptide-1(7-36)amide (GLP-1) is a 30-residue peptide hormone released from intestinal L cells following nutrient consumption. It potentiates the glucose-induced secretion of insulin from pancreatic beta cells, increases insulin expression, inhibits beta-cell apoptosis, promotes beta-cell neogenesis, reduces glucagon secretion, delays gastric emptying, promotes satiety and increases peripheral glucose disposal. These multiple effects have generated a great deal of interest in the discovery of long-lasting agonists of the GLP-1 receptor (GLP-1R) in order to treat type 2 diabetes. This review article summarizes the literature regarding the discovery of GLP-1 and its physiological functions. The structure, function and sequence-activity relationships of the hormone and its natural analogue exendin-4 (Ex4) are reviewed in detail. The current knowledge of the structure of GLP-1R, a Family B GPCR, is summarized and discussed, before its known interactions with the principle peptide ligands are described and summarized. Finally, progress in discovering non-peptide ligands of GLP-1R is reviewed. GLP-1 is clearly an important hormone linking nutrient consumption with blood sugar control, and therefore knowledge of its structure, function and mechanism of action is of great importance.
© 2011 The Author. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

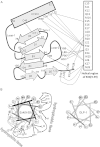
References
-
- Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O. Structure-activity studies of glucagon-like peptide-1. J Biol Chem. 1994;269:6275–6278. - PubMed
-
- Ahrén B. GLP-1 for type 2 diabetes. Exp Cell Res. 2011;317:1239–1245. - PubMed
-
- Al-Sabah S, Donnelly D. The positive charge at Lys-288 of the glucagon-like peptide-1 (GLP-1) receptor is important for binding the N-terminus of peptide agonists. FEBS Lett. 2003b;553:342–346. - PubMed
-
- Al-Sabah S, Donnelly D. The primary ligand-binding interaction at the GLP-1 receptor is via the putative helix of the peptide agonists. Protein Pept Lett. 2004;11:9–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

