Simultaneous optical mapping of transmembrane potential and wall motion in isolated, perfused whole hearts
- PMID: 21950934
- PMCID: PMC3194792
- DOI: 10.1117/1.3630115
Simultaneous optical mapping of transmembrane potential and wall motion in isolated, perfused whole hearts
Abstract
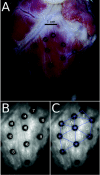
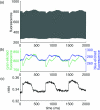
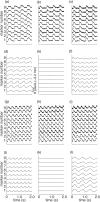
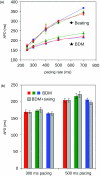
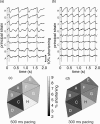
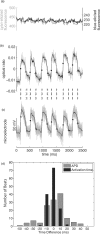
Optical mapping of cardiac propagation has traditionally been hampered by motion artifact, chiefly due to changes in photodetector-to-tissue registration as the heart moves. We have developed an optical mapping technique to simultaneously record electrical waves and mechanical contraction in isolated hearts. This allows removal of motion artifact from transmembrane potential (V(m)) recordings without the use of electromechanical uncoupling agents and allows the interplay of electrical and mechanical events to be studied at the whole organ level. Hearts are stained with the voltage-sensitive dye di-4-ANEPPS and ring-shaped markers are attached to the epicardium. Fluorescence, elicited on alternate frames by 450 and 505 nm light-emitting diodes, is recorded at 700 frames∕ per second by a camera fitted with a 605 ± 25 nm emission filter. Marker positions are tracked in software. A signal, consisting of the temporally interlaced 450 and 505 nm fluorescence, is collected from the pixels enclosed by each moving ring. After deinterlacing, the 505 nm signal consists of V(m) with motion artifact, while the 450 nm signal is minimally voltage-sensitive and contains primarily artifacts. The ratio of the two signals estimates V(m). Deformation of the tissue enclosed by each set of 3 rings is quantified using homogeneous finite strain.
Figures




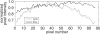
References
-
- Shenasa M., Hendricks G., Borggrefe B., and Breithardt G., Cardiac Mapping. 3rd Ed., Blackwell Publishing, Oxford, United Kingdom: (2009).
-
- Qin H., Kay M. W., Chattipakorn N., Redden D. T., Ideker R. E., and Rogers J. M., “Effects of heart isolation, voltage-sensitive dye, and electromechanical uncoupling agents on ventricular fibrillation,” Am. J. Physiol. Heart Circ. Physiol. 284, H1818–H1826 (2003).10.1152/ajpheart.00923.2002 - DOI - PubMed
-
- Schiller N. B., Shah P. M., Crawford M., DeMaria A., Devereux R., Feigenbaum H., Gutgesell H., Reichek N., Sahn D., Schnittger I., Silverman N. H., and Tajik A. J., “Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms,” J. Am. Soc. Echocardiogr. 2, 358–367 (1989). - PubMed

