Zebrafish models of germ cell tumor
- PMID: 21951524
- PMCID: PMC3932324
- DOI: 10.1016/B978-0-12-381320-6.00001-1
Zebrafish models of germ cell tumor
Abstract
Germ cell tumors are neoplasms arising from pluripotent germ cells. In humans, these tumors occur in infants, children and young adults. The tumors display a wide range of histologic differentiation states which exhibit different clinical behaviors. Information about the molecular basis of germ cell tumors, and representative animal models of these neoplasms, are lacking. Germline development in zebrafish and humans is broadly conserved, making the fish a useful model to probe the connections between germ cell development and tumorigenesis. Here, we provide an overview of germline development and a brief review of germ cell tumor biology in humans and zebrafish. We also outline some methods for studying the zebrafish germline.
Copyright © 2011 Elsevier Inc. All rights reserved.
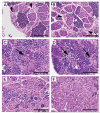
Figures

References
-
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T, Surani MA. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;8:623–30. - PubMed
-
- Anderson R, Copeland TK, Scholer H, Heasman J, Wylie C. The onset of germ cell migration in the mouse embryo. Mech Dev. 2000;91:61–8. - PubMed
-
- Anderson R, Fassler R, Georges-Labouesse E, Hynes RO, Bader BL, Kreidberg JA, Schaible K, Heasman J, Wylie C. Mouse primordial germ cells lacking beta1 integrins enter the germline but fail to migrate normally to the gonads. Development. 1999a;126:1655–64. - PubMed
-
- Anderson R, Schaible K, Heasman J, Wylie C. Expression of the homophilic adhesion molecule, Ep-CAM, in the mammalian germ line. J Reprod Fertil. 1999b;116:379–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

