The role of amputation as an outcome measure in cellular therapy for critical limb ischemia: implications for clinical trial design
- PMID: 21951607
- PMCID: PMC3191337
- DOI: 10.1186/1479-5876-9-165
The role of amputation as an outcome measure in cellular therapy for critical limb ischemia: implications for clinical trial design
Abstract
Background: Autologous bone marrow-derived stem cells have been ascribed an important therapeutic role in No-Option Critical limb Ischemia (NO-CLI). One primary endpoint for evaluating NO-CLI therapy is major amputation (AMP), which is usually combined with mortality for AMP-free survival (AFS). Only a trial which is double blinded can eliminate physician and patient bias as to the timing and reason for AMP. We examined factors influencing AMP in a prospective double-blinded pilot RCT (2:1 therapy to control) of 48 patients treated with site of service obtained bone marrow cells (BMAC) as well as a systematic review of the literature.
Methods: Cells were injected intramuscularly in the CLI limbs as either BMAC or placebo (peripheral blood). Six month AMP rates were compared between the two arms. Both patient and treating team were blinded of the assignment in follow-up examinations. A search of the literature identified 9 NO-CLI trials, the control arms of which were used to determine 6 month AMP rates and the influence of tissue loss.
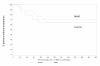
Results: Fifteen amputations occurred during the 6 month period, 86.7% of these during the first 4 months. One amputation occurred in a Rutherford 4 patient. The difference in amputation rate between patients with rest pain (5.6%) and those with tissue loss (46.7%), irrespective of treatment group, was significant (p = 0.0029). In patients with tissue loss, treatment with BMAC demonstrated a lower amputation rate than placebo (39.1% vs. 71.4%, p = 0.1337). The Kaplan-Meier time to amputation was longer in the BMAC group than in the placebo group (p = 0.067). Projecting these results to a pivotal trial, a bootstrap simulation model showed significant difference in AFS between BMAC and placebo with a power of 95% for a sample size of 210 patients. Meta-analysis of the literature confirmed a difference in amputation rate between patients with tissue loss and rest pain.
Conclusions: BMAC shows promise in improving AMP-free survival if the trends in this pilot study are validated in a larger pivotal trial. The difference in amp rate between Rutherford 4 & 5 patients suggests that these patients should be stratified in future RCTs.
Figures



References
-
- Nowygrod R, Egorova N, Greco G, Anderson P, Gelijns A, Moskowitz A, McKinsey J, Morrissey N, Kent KC. Trends, complications, and mortality in peripheral vascular surgery. J Vasc Surg. 2006;43:205–216. - PubMed
-
- Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. - PubMed
-
- Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. - PubMed
-
- Iafrati MD, Hallett JW, Geils G, Lumsden AB, Peden EK, Bandyk DF, Pearl G, Raghavan V, Ascher E, Hingorani A, Bone marrow aspirate concentrate in critical limb ischemia: Early results and lessons learned from a multicenter randomized double blind trial. J Vasc Surg. 2011. in press . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

