Erythrocyte membrane changes of chorea-acanthocytosis are the result of altered Lyn kinase activity
- PMID: 21951684
- PMCID: PMC3217364
- DOI: 10.1182/blood-2011-05-355339
Erythrocyte membrane changes of chorea-acanthocytosis are the result of altered Lyn kinase activity
Abstract
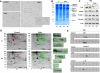
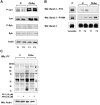
Acanthocytic RBCs are a peculiar diagnostic feature of chorea-acanthocytosis (ChAc), a rare autosomal recessive neurodegenerative disorder. Although recent years have witnessed some progress in the molecular characterization of ChAc, the mechanism(s) responsible for generation of acanthocytes in ChAc is largely unknown. As the membrane protein composition of ChAc RBCs is similar to that of normal RBCs, we evaluated the tyrosine (Tyr)-phosphorylation profile of RBCs using comparative proteomics. Increased Tyr phosphorylation state of several membrane proteins, including band 3, β-spectrin, and adducin, was noted in ChAc RBCs. In particular, band 3 was highly phosphorylated on the Tyr-904 residue, a functional target of Lyn, but not on Tyr-8, a functional target of Syk. In ChAc RBCs, band 3 Tyr phosphorylation by Lyn was independent of the canonical Syk-mediated pathway. The ChAc-associated alterations in RBC membrane protein organization appear to be the result of increased Tyr phosphorylation leading to altered linkage of band 3 to the junctional complexes involved in anchoring the membrane to the cytoskeleton as supported by coimmunoprecipitation of β-adducin with band 3 only in ChAc RBC-membrane treated with the Lyn-inhibitor PP2. We propose this altered association between membrane skeleton and membrane proteins as novel mechanism in the generation of acanthocytes in ChAc.
Figures




References
-
- Danek A, Jung HH, Melone MA, Rampoldi L, Broccoli V, Walker RH. Neuroacanthocytosis: new developments in a neglected group of dementing disorders. J Neurol Sci. 2005;229–230:171–186. - PubMed
-
- Danek A, Walker RH. Neuroacanthocytosis. Curr Opin Neurol. 2005;18(4):386–392. - PubMed
-
- Jung HH, Danek A, Frey BM. McLeod syndrome: a neurohaematological disorder. Vox Sang. 2007;93(2):112–121. - PubMed
-
- Rampoldi L, Danek A, Monaco AP. Clinical features and molecular bases of neuroacanthocytosis. J Mol Med. 2002;80(8):475–491. - PubMed
-
- Velayos-Baeza A, Levecque C, Dobson-Stone C, Monaco AP. The function of chorein. In: Walker RH, Saiki S, Danek A, editors. Neuro-acanthocytosis Syndromes II. Berlin, Germany: Springer-Verlag; 2008. pp. 87–105.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

