Movement based artifacts may contaminate extracellular electrical recordings from GI muscles
- PMID: 21951699
- PMCID: PMC4793914
- DOI: 10.1111/j.1365-2982.2011.01784.x
Movement based artifacts may contaminate extracellular electrical recordings from GI muscles
Abstract
Background: Electrical slow waves drive peristaltic contractions in the stomach and facilitate gastric emptying. In gastroparesis and other disorders associated with altered gastric emptying, motility defects have been related to altered slow wave frequency and disordered propagation. Experimental and clinical measurements of slow waves are made with extracellular or abdominal surface recording.
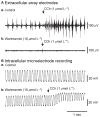
Methods: We tested the consequences of muscle contractions and movement on biopotentials recorded from murine gastric muscles with array electrodes and pairs of silver electrodes.
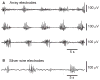
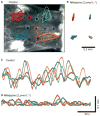
Key results: Propagating biopotentials were readily recorded from gastric sheets composed of the entire murine stomach. The biopotentials were completely blocked by nifedipine (2 μmol L(-1) ) that blocked contractile movements and peristaltic contractions. Wortmannin, an inhibitor of myosin light chain kinase, also blocked contractions and biopotentials. Stimulation of muscles with carbachol increased the frequency of biopotentials in control conditions but failed to elicit biopotentials with nifedipine or wortmannin present. Intracellular recording with microelectrodes showed that authentic gastric slow waves occur at a faster frequency typically than biopotentials recorded with extracellular electrodes, and electrical slow waves recorded with intracellular electrodes were unaffected by suppression of movement. Electrical transients, equal in amplitude to biopotentials recorded with extracellular electrodes, were induced by movements produced by small transient stretches (<1 mm) of paralyzed or formalin fixed gastric sheets.
Conclusions & inferences: These data demonstrate significant movement artifacts in extracellular recordings of biopotentials from murine gastric muscles and suggest that movement suppression should be an obligatory control when monitoring electrical activity and characterizing propagation and coordination of electrical events with extracellular recording techniques.
© 2011 Blackwell Publishing Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
There are no conflicts of interest to disclose.
Figures







Comment in
-
Frequency analysis may distinguish the effects of calcium antagonists on mechanical and electrical activity.Neurogastroenterol Motil. 2012 Apr;24(4):397; author reply 398. doi: 10.1111/j.1365-2982.2012.01882.x. Neurogastroenterol Motil. 2012. PMID: 22414186 No abstract available.
References
-
- Alvarez WC, Mahoney LJ. Action current in stomach and intestine. Am J Physiol. 1922;58:476–93.
-
- Bortoff A. Slow potential variation of small intestine. Am J Physiol. 1961;201:203–8.
-
- Kobayashi M, Prosser CL, Nagai T. Electrical properties of intestinal muscle as measured intracellularly and extracellularly. Am J Physiol. 1967;213:275–86. - PubMed
-
- Bortoff A. Configuration of intestinal slow waves obtained by monopolar recording techniques. Am J Physiol. 1967;213:157–62. - PubMed
-
- Boev K, Golenhofen K. Sucrose-gap technique with pressed-rubber membranes. PflugersArch. 1974;349:277–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

