High-speed MALDI-TOF imaging mass spectrometry: rapid ion image acquisition and considerations for next generation instrumentation
- PMID: 21953043
- PMCID: PMC3514015
- DOI: 10.1007/s13361-011-0121-0
High-speed MALDI-TOF imaging mass spectrometry: rapid ion image acquisition and considerations for next generation instrumentation
Abstract
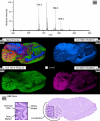
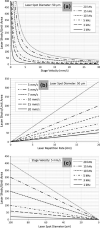
A prototype matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometer has been used for high-speed ion image acquisition. The instrument incorporates a Nd:YLF solid state laser capable of pulse repetition rates up to 5 kHz and continuous laser raster sampling for high-throughput data collection. Lipid ion images of a sagittal rat brain tissue section were collected in 10 min with an effective acquisition rate of roughly 30 pixels/s. These results represent more than a 10-fold increase in throughput compared with current commercially available instrumentation. Experiments aimed at improving conditions for continuous laser raster sampling for imaging are reported, highlighting proper laser repetition rates and stage velocities to avoid signal degradation from significant oversampling. As new high spatial resolution and large sample area applications present themselves, the development of high-speed microprobe MALDI imaging mass spectrometry is essential to meet the needs of those seeking new technologies for rapid molecular imaging.
Figures


References
-
- Caprioli RM, Farmer TB, Gile J. Molecular Imaging of Biological Samples: Localization of Peptides and Proteins using MALDI-TOF MS. Anal. Chem. 1997;69:4751–4760. - PubMed
-
- Amstalden van Hove ER, Smith DF, Heeren RMA. A Concise Review of Mass Spectrometry Imaging. J. Chromatogr. A. 2010;1217:3946–3954. - PubMed
-
- McDonnell LA, Corthals GL, Willems SM, van Remoortere A, van Zeijl RJM, Deelder AM. Peptide and Protein Imaging Mass Spectrometry in Cancer Research. Journal of Proteomics. 2010;73(10):1921–1944. - PubMed
-
- Svatos A. Mass Spectrometric Imaging of Small Molecules. Trends Biotechnol. 2010;28:425–434. - PubMed
-
- Cornett DS, Reyzer ML, Chaurand P, Caprioli RM. MALDI Imaging Mass Spectrometry: Molecular Snapshots of Biochemical Systems. Nat. Methods. 2007;4:828–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

