Efficacy of neoadjuvant therapy with trastuzumab concurrent with anthracycline- and nonanthracycline-based regimens for HER2-positive breast cancer
- PMID: 21953213
- PMCID: PMC3274632
- DOI: 10.1002/cncr.26555
Efficacy of neoadjuvant therapy with trastuzumab concurrent with anthracycline- and nonanthracycline-based regimens for HER2-positive breast cancer
Abstract
Background: The aim of this study was to evaluate the pathologic complete response (pCR) rates and relapse-free survival (RFS) and overall survival (OS) of patients receiving neoadjuvant systemic therapy (NST) with trastuzumab in combination with an anthracycline- or nonanthracycline-based regimen.
Methods: In this retrospective nonrandomized study, the authors reviewed records of 300 patients with HER2-positive breast cancer treated with either sequential paclitaxel and trastuzumab and FEC75 in combination with trastuzumab (PH-FECH) or docetaxel, carboplatin, and trastuzumab (TCH). The Kaplan-Meier product-limit method was used to estimate RFS and OS rates. Logistic regression models and Cox proportional hazards models were fit to determine the associations between NST, pCR, and survival.
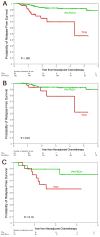
Results: There was no significant difference in the decline in cardiac ejection fraction; however, patients who received PH-FECH had fewer cardiac comorbidities at baseline (P = .002). pCR rates were 60.6% and 43.3% for patients who received PH-FECH (n = 235) and TCH (n = 65), respectively (P = .016). Patients who received PH-FECH were 1.45 times more likely to have a pCR (odds ratio [OR], 1.45; 95% confidence interval [CI], 1.06-1.98; P = .02). Three-year RFS rates were 93% and 71% (P < .001), and 3-year OS rates were 96% and 86% (P = .008) for patients who received PH-FECH and TCH, respectively. Patients who received PH-FECH had a lower risk of recurrence (hazard ratio [HR], 0.27; 95% CI, 0.12-0.60; P = .001) and death (HR, 0.37; 95% CI, 0.12-1.13; P = .08) than those treated with TCH.
Conclusions: The type of NST in HER2-positive breast cancer is predictive of pCR rate independent of disease and patient characteristics. Although TCH is active, PH-FECH shows a higher pCR rate and RFS advantage.
Copyright © 2011 American Cancer Society.
Figures
References
-
- Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17(2):460–469. - PubMed
-
- Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg. 1995;180(3):297–306. - PubMed
-
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–2685. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous