LC-MS(n) analysis of isomeric chondroitin sulfate oligosaccharides using a chemical derivatization strategy
- PMID: 21953261
- PMCID: PMC3187560
- DOI: 10.1007/s13361-011-0174-0
LC-MS(n) analysis of isomeric chondroitin sulfate oligosaccharides using a chemical derivatization strategy
Abstract
Improved methods for structural analyses of glycosaminoglycans (GAGs) are required to understand their functional roles in various biological processes. Major challenges in structural characterization of complex GAG oligosaccharides using liquid chromatography-mass spectrometry (LC-MS) include the accurate determination of the patterns of sulfation due to gas-phase losses of the sulfate groups upon collisional activation and inefficient on-line separation of positional sulfation isomers prior to MS/MS analyses. Here, a sequential chemical derivatization procedure including permethylation, desulfation, and acetylation was demonstrated to enable both on-line LC separation of isomeric mixtures of chondroitin sulfate (CS) oligosaccharides and accurate determination of sites of sulfation by MS(n). The derivatized oligosaccharides have sulfate groups replaced with acetyl groups, which are sufficiently stable to survive MS(n) fragmentation and reflect the original sulfation patterns. A standard reversed-phase LC-MS system with a capillary C18 column was used for separation, and MS(n) experiments using collision-induced dissociation (CID) were performed. Our results indicate that the combination of this derivatization strategy and MS(n) methodology enables accurate identification of the sulfation isomers of CS hexasaccharides with either saturated or unsaturated nonreducing ends. Moreover, derivatized CS hexasaccharide isomer mixtures become separable by LC-MS method due to different positions of acetyl modifications.
Figures


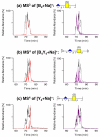
 ΔGlcA,
ΔGlcA,  GlcA,
GlcA,  GalNAc. All product ions labeled on the spectra are singly charged and monosodiated unless otherwise annotated
GalNAc. All product ions labeled on the spectra are singly charged and monosodiated unless otherwise annotated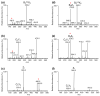

References
-
- Perrimon N, Bernfield M. Cellular functions of proteoglycans-an overview. Semin. Cell Dev. Biol. 2001;12:65–67. - PubMed
-
- Deepa SS, Yamada S, Fukui S, Sugahara K. Structural determination of novel sulfated octasaccharides isolated from chondroitin sulfate of shark cartilage and their application for characterizing monoclonal antibody epitopes. Glycobiology. 2007;17:631–645. - PubMed
-
- Whitelock JM, Iozzo RV. Heparan sulfate: a complex polymer charged with biological activity. Chem. Rev. 2005;105:2745–2764. - PubMed
-
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev. 2000;80:1267–1290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

