The Fbw7 tumor suppressor regulates nuclear factor E2-related factor 1 transcription factor turnover through proteasome-mediated proteolysis
- PMID: 21953459
- PMCID: PMC3234752
- DOI: 10.1074/jbc.M111.253807
The Fbw7 tumor suppressor regulates nuclear factor E2-related factor 1 transcription factor turnover through proteasome-mediated proteolysis
Abstract
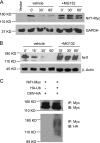
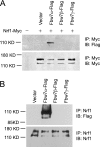
Nuclear factor E2-related factor 1 (Nrf1) is a basic leucine zipper transcription factor that plays important roles in cellular stress response and development. Currently, the mechanism regulating Nrf1 expression is poorly understood. We report here that Nrf1 is a short-lived protein that is targeted by F-box protein Fbw7, which is the substrate-specifying component of SCF (Skp1-Cul1-Fbox protein-Rbx1)-type ubiquitin ligase for degradation via the ubiquitin-proteasome pathway. We show that Fbw7 directly binds Nrf1 through a Cdc4 phosphodegron and that enforced expression of Fbw7 promotes the ubiquitination and degradation of Nrf1. Conversely, depletion of endogenous Fbw7 leads to decreased Nrf1 ubiquitination and accumulation of Nrf1 protein. Accordingly, expression of Fbw7 leads to down-regulation of antioxidant response element-driven gene activation, whereas disruption of Fbw7-mediated destabilization of Nrf1 leads to increased antioxidant response element-driven gene expression. Together, these data identify Fbw7 as a regulator of Nrf1 expression and reveal a novel function of Fbw7 in cellular stress response.
Figures



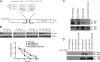
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

