CREMα suppresses spleen tyrosine kinase expression in normal but not systemic lupus erythematosus T cells
- PMID: 21953500
- PMCID: PMC3250560
- DOI: 10.1002/art.33375
CREMα suppresses spleen tyrosine kinase expression in normal but not systemic lupus erythematosus T cells
Abstract
Objective: T cells from patients with systemic lupus erythematosus (SLE) display increased amounts of spleen tyrosine kinase (Syk), which is involved in the aberrant CD3/T cell receptor-mediated signaling process, and increased amounts of CREMα, which suppresses the production of interleukin-2. Syk expression can be suppressed by CREMα. This study was undertaken to investigate why CREMα fails to suppress Syk expression in SLE T cells.
Methods: CREMα was overexpressed in healthy T cells by transfection with CREMα expression vector, and Syk expression and phosphorylation were measured. A newly identified cAMP response element (CRE) site on the SYK promoter was characterized by chromatin immunoprecipitation (ChIP) and electrophoretic mobility shift assay. The CREMα-mediated repression of Syk expression was further evaluated by analyzing SYK promoter activity. T cells from SLE patients and healthy individuals were subjected to ChIP to evaluate CREMα binding and histone H3 acetylation.
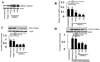
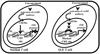
Results: Increased CREMα levels suppressed Syk expression by direct binding to a CRE site of the SYK promoter in T cells from healthy individuals but failed to do so in T cells from SLE patients. The failure of CREMα to suppress Syk expression in SLE T cells was due to weaker binding to the CRE site of the SYK promoter compared to healthy T cells because the promoter site is hypoacetylated in SLE T cells and therefore of limited access to transcription factors.
Conclusion: Our findings indicate that epigenetic alteration of the SYK promoter in SLE T cells results in the inability of the transcriptional repressor CREMα to bind and suppress the expression of Syk, resulting in aberrant T cell signaling.
Copyright © 2012 by the American College of Rheumatology.
Conflict of interest statement
Figures



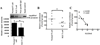
References
-
- Rudd CE. Adaptors and molecular scaffolds in immune cell signaling. Cell. 1999;96(1):5–8. - PubMed
-
- Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of SYK protein-tyrosine kinase. J Biochem. 2001;130(2):177–186. - PubMed
-
- Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23(48):7990–8000. - PubMed
-
- Krishnan S, Warke VG, Nambiar MP, Tsokos GC, Farber DL. The FcR gamma subunit and SYK kinase replace the CD3 zeta-chain and ZAP-70 kinase in the TCR signaling complex of human effector CD4 T cells. J Immunol. 2003;170(8):4189–4195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

