Prospectively determined impact of type 1 diabetes on brain volume during development
- PMID: 21953611
- PMCID: PMC3198062
- DOI: 10.2337/db11-0589
Prospectively determined impact of type 1 diabetes on brain volume during development
Abstract
Objective: The impact of type 1 diabetes mellitus (T1DM) on the developing central nervous system is not well understood. Cross-sectional, retrospective studies suggest that exposure to glycemic extremes during development is harmful to brain structure in youth with T1DM. However, these studies cannot identify brain regions that change differentially over time depending on the degree of exposure to glycemic extremes.
Research design and methods: We performed a longitudinal, prospective structural neuroimaging study of youth with T1DM (n = 75; mean age = 12.5 years) and their nondiabetic siblings (n = 25; mean age = 12.5 years). Each participant was scanned twice, separated by 2 years. Blood glucose control measurements (HbA(1c), glucose meter results, and reports of severe hypoglycemia) were acquired during the 2-year follow-up. Sophisticated image registration algorithms were performed, followed by whole brain and voxel-wise statistical analyses of the change in gray and white matter volume, controlling for age, sex, and age of diabetes onset.
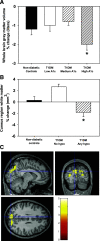
Results: The T1DM and nondiabetic control (NDC) sibling groups did not differ in whole brain or voxel-wise change over the 2-year follow-up. However, within the T1DM group, participants with more hyperglycemia had a greater decrease in whole brain gray matter compared with those with less hyperglycemia (P < 0.05). Participants who experienced severe hypoglycemia had greater decreases in occipital/parietal white matter volume compared with those with no severe hypoglycemia (P < 0.05) and compared with the NDC sibling group (P < 0.05).
Conclusions: These results demonstrate that within diabetes, exposure to hyperglycemia and severe hypoglycemia may result in subtle deviation from normal developmental trajectories of the brain.
Trial registration: ClinicalTrials.gov NCT00879203.
Figures
References
-
- Olsen BS, Sjølie A, Hougaard P, et al. . A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. J Diabetes Complications 2000;14:295–300 - PubMed
-
- Chkhartishvili D, Khachapuridze N, Natriashvili G, Geladze N, Kapanadze N. Nerve conduction abnormalities in children with type I diabetes. Annals of Biomedical Research and Education 2002;2:331–334
-
- Riihimaa PH, Suominen K, Tolonen U, Jäntti V, Knip M, Tapanainen P. Peripheral nerve function is increasingly impaired during puberty in adolescents with type 1 diabetes. Diabetes Care 2001;24:1087–1092 - PubMed
-
- Perantie DC, Wu J, Koller JM, et al. . Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care 2007;30:2331–2337 - PubMed

