Asymmetric total synthesis of the epoxykinamycin FL-120 B'
- PMID: 21953671
- PMCID: PMC3477604
- DOI: 10.1002/anie.201104504
Asymmetric total synthesis of the epoxykinamycin FL-120 B'
Abstract
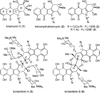
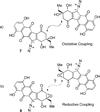
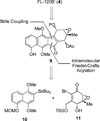
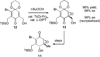
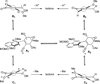
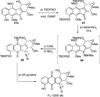
Turn up the heat: An asymmetric total synthesis of the epoxykinamycin FL-120B’ is reported. The synthesis establishes a route to epoxide-containing diazobenzofluorenes which could potentially serve as monomers to the dimeric lomaiviticins. Key steps include Sharpless asymmetric epoxidation, Stille coupling, and intramolecular Friedel-Crafts acylation of atropisomeric carboxylic acids at elevated temperatures to construct the FL-120B’ core structure.
Figures


References
-
-
For a recent review on diazo natural products, see: Nawrat CC, Moody CJ. Nat. Prod. Rep. 2011;28:1426–1444.
-
-
-
For a review on the biosynthesis of diazobenzofluorene natural products, see: Gould SJ. Chem. Rev. 1997;97:2499–2509.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

