Outer hair cell-specific prestin-CreERT2 knockin mouse lines
- PMID: 21954035
- PMCID: PMC3261330
- DOI: 10.1002/dvg.20810
Outer hair cell-specific prestin-CreERT2 knockin mouse lines
Abstract
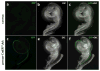
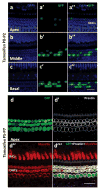
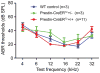
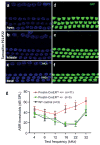
Outer hair cells (OHCs) in the cochlea are crucial for the remarkable hearing sensitivity and frequency tuning. To understand OHC physiology and pathology, it is imperative to use mouse genetic tools to manipulate gene expression specifically in OHCs. Here, we generated two prestin knockin mouse lines: (1) the prestin-CreERT2 line, with an internal ribosome entry site-CreERT2-FRT-Neo-FRT cassette inserted into the prestin locus after the stop codon, and (2) the prestin-CreERT2-NN line, with the FRT-Neo-FRT removed subsequently. We characterized the inducible Cre activity of both lines by crossing them with the reporter lines CAG-eGFP and Ai6. Cre activity was induced with tamoxifen at various postnatal ages and only detected in OHCs, resembling the endogenous prestin expression pattern. Moreover, prestin-CreERT2+/-(heterozygotes) and +/+(homozygotes) as well as prestin-CreERT2-NN+/-mice displayed normal hearing. These two prestin-CreERT2 mouse lines are therefore useful tools to analyze gene function in OHCs in vivo.
Copyright © 2011 Wiley Periodicals, Inc.
Figures

References
-
- Adler HJ, Belyantseva IA, Merritt RC, Jr, Frolenkov GI, Dougherty GW, Kachar B. Expression of prestin, a membrane motor protein, in the mammalian auditory and vestibular periphery. Hear Res. 2003;184:27–40. - PubMed
-
- Chow LM, Tian Y, Weber T, Corbett M, Zuo J, Baker SJ. Inducible Cre recombinase activity in mouse cerebellar granule cell precursors and inner ear hair cells. Dev Dyn. 2006;235:2991–2998. - PubMed
-
- Davis RR, Kuo MW, Stanton SG, Canlon B, Krieg E, Alagramam KN. N-Acetyl L-cysteine does not protect against premature age-related hearing loss in C57BL/6J mice: a pilot study. Hear Res. 2007;226:203–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

