The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction
- PMID: 21954467
- PMCID: PMC3203450
- DOI: 10.1105/tpc.111.089581
The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction
Erratum in
- Plant Cell. 2011 Dec;23(12):4526
Abstract
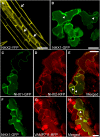
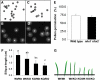
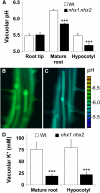
Intracellular Na(+)/H(+) (NHX) antiporters have important roles in cellular pH and Na(+), K(+) homeostasis. The six Arabidopsis thaliana intracellular NHX members are divided into two groups, endosomal (NHX5 and NHX6) and vacuolar (NHX1 to NHX4). Of the vacuolar members, NHX1 has been characterized functionally, but the remaining members have largely unknown roles. Using reverse genetics, we show that, unlike the single knockouts nhx1 or nhx2, the double knockout nhx1 nhx2 had significantly reduced growth, smaller cells, shorter hypocotyls in etiolated seedlings and abnormal stamens in mature flowers. Filaments of nhx1 nhx2 did not elongate and lacked the ability to dehisce and release pollen, resulting in a near lack of silique formation. Pollen viability and germination was not affected. Quantification of vacuolar pH and intravacuolar K(+) concentrations indicated that nhx1 nhx2 vacuoles were more acidic and accumulated only 30% of the wild-type K(+) concentration, highlighting the roles of NHX1 and NHX2 in mediating vacuolar K(+)/H(+) exchange. Growth under added Na(+), but not K(+), partly rescued the flower and growth phenotypes. Our results demonstrate the roles of NHX1 and NHX2 in regulating intravacuolar K(+) and pH, which are essential to cell expansion and flower development.
Figures





Comment in
-
Functional analysis of Arabidopsis NHX antiporters: the role of the vacuole in cellular turgor and growth.Plant Cell. 2011 Sep;23(9):3087-8. doi: 10.1105/tpc.111.230914. Epub 2011 Sep 27. Plant Cell. 2011. PMID: 21954465 Free PMC article. No abstract available.
References
-
- Aharon G.S., Apse M.P., Duan S., Hua X., Blumwald E. (2003). Characterization of a family of vacuolar Na+/H+ antiporters in Arabidopsis thaliana. Plant Soil 253: 245–256
-
- Alandete-Saez M., Ron M., McCormick S. (2008). GEX3, expressed in the male gametophyte and in the egg cell of Arabidopsis thaliana, is essential for micropylar pollen tube guidance and plays a role during early embryogenesis. Molecular Plant 1: 586–598 - PubMed
-
- Amtmann A., Leigh R. (2010). Ion homeostasis. Abiotic Stress Adaptation in Plants, Pareek A., Sopory S.K., Bohnert H.J., (Dordrecht, The Netherlands: Springer; ), pp. 245–262
-
- Apse M.P., Blumwald E. (2007). Na+ transport in plants. FEBS Lett. 581: 2247–2254 - PubMed
-
- Apse M.P., Sottosanto J.B., Blumwald E. (2003). Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 36: 229–239 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

