Tunable self-assembly of genetically engineered silk--elastin-like protein polymers
- PMID: 21955178
- PMCID: PMC3224811
- DOI: 10.1021/bm201165h
Tunable self-assembly of genetically engineered silk--elastin-like protein polymers
Abstract
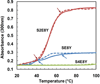
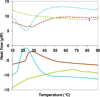
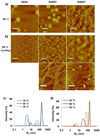
Silk--elastin-like protein polymers (SELPs), consisting of the repeating units of silk and elastin blocks, combine a set of outstanding physical and biological properties of silk and elastin. Because of the unique properties, SELPs have been widely fabricated into various materials for the applications in drug delivery and tissue engineering. However, little is known about the fundamental self-assembly characteristics of these remarkable polymers. Here we propose a two-step self-assembly process of SELPs in aqueous solution for the first time and report the importance of the ratio of silk-to-elastin blocks in a SELP's repeating unit on the assembly of the SELP. Through precise tuning of the ratio of silk to elastin, various structures including nanoparticles, hydrogels, and nanofibers could be generated either reversibly or irreversibly. This assembly process might provide opportunities to generate innovative smart materials for biosensors, tissue engineering, and drug delivery. Furthermore, the newly developed SELPs in this study may be potentially useful as biomaterials for controlled drug delivery and biomedical engineering.
Figures



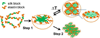
Similar articles
-
Bioengineered elastin- and silk-biomaterials for drug and gene delivery.Adv Drug Deliv Rev. 2020;160:186-198. doi: 10.1016/j.addr.2020.10.008. Epub 2020 Oct 17. Adv Drug Deliv Rev. 2020. PMID: 33080258 Free PMC article.
-
Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics.Expert Opin Drug Deliv. 2015 May;12(5):779-91. doi: 10.1517/17425247.2015.989830. Epub 2014 Dec 5. Expert Opin Drug Deliv. 2015. PMID: 25476201 Free PMC article. Review.
-
Rationally Designed Redox-Sensitive Protein Hydrogels with Tunable Mechanical Properties.Biomacromolecules. 2016 Nov 14;17(11):3508-3515. doi: 10.1021/acs.biomac.6b00973. Epub 2016 Oct 11. Biomacromolecules. 2016. PMID: 27700059
-
Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery.Biomacromolecules. 2014 Mar 10;15(3):908-14. doi: 10.1021/bm4017594. Epub 2014 Feb 21. Biomacromolecules. 2014. PMID: 24527851 Free PMC article.
-
Engineering aqueous fiber assembly into silk-elastin-like protein polymers.Macromol Rapid Commun. 2014 Jul;35(14):1273-9. doi: 10.1002/marc.201400058. Epub 2014 May 3. Macromol Rapid Commun. 2014. PMID: 24798978
Cited by
-
Mechanism of resilin elasticity.Nat Commun. 2012;3:1003. doi: 10.1038/ncomms2004. Nat Commun. 2012. PMID: 22893127 Free PMC article.
-
Silk-Elastin-Like-Protein/Graphene-Oxide Composites for Dynamic Electronic Biomaterials.Macromol Biosci. 2022 Aug;22(8):e2200122. doi: 10.1002/mabi.202200122. Epub 2022 Jun 24. Macromol Biosci. 2022. PMID: 35634798 Free PMC article.
-
Inducing an LCST in hydrophilic polysaccharides via engineered macromolecular hydrophobicity.Sci Rep. 2023 Sep 9;13(1):14896. doi: 10.1038/s41598-023-41947-z. Sci Rep. 2023. PMID: 37689784 Free PMC article.
-
Protein-Engineered Functional Materials.Adv Healthc Mater. 2019 Jun;8(11):e1801374. doi: 10.1002/adhm.201801374. Epub 2019 Apr 2. Adv Healthc Mater. 2019. PMID: 30938924 Free PMC article. Review.
-
Bioengineered elastin- and silk-biomaterials for drug and gene delivery.Adv Drug Deliv Rev. 2020;160:186-198. doi: 10.1016/j.addr.2020.10.008. Epub 2020 Oct 17. Adv Drug Deliv Rev. 2020. PMID: 33080258 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

