Thiocolchicoside suppresses osteoclastogenesis induced by RANKL and cancer cells through inhibition of inflammatory pathways: a new use for an old drug
- PMID: 21955206
- PMCID: PMC3413851
- DOI: 10.1111/j.1476-5381.2011.01702.x
Thiocolchicoside suppresses osteoclastogenesis induced by RANKL and cancer cells through inhibition of inflammatory pathways: a new use for an old drug
Abstract
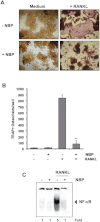
Background and purpose: Most patients with cancer die not because of the tumour in the primary site, but because it has spread to other sites. Common tumours, such as breast, multiple myeloma, and prostate tumours, frequently metastasize to the bone. To search for an inhibitor of cancer-induced bone loss, we investigated the effect of thiocolchicoside, a semi-synthetic colchicoside derived from the plant Gloriosa superba and clinically used as a muscle relaxant, on osteoclastogenesis induced by receptor activator of NF-κB ligand (RANKL) and tumour cells.
Experimental approach: We used RAW 264.7 (murine macrophage) cells, a well-established system for osteoclastogenesis, and evaluated the effect of thiocolchicoside on RANKL-induced NF-κB signalling and osteoclastogenesis as well as on osteoclastogenesis induced by tumour cells.
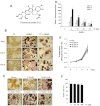
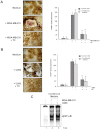
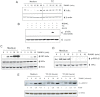
Key results: Thiocolchicoside suppressed osteoclastogenesis induced by RANKL, and by breast cancer and multiple myeloma cells. Inhibition of the NF-κB pathway was responsible for this effect since the colchicoside inhibited RANKL-induced NF-κB activation, activation of IκB kinase (IKK) and suppressed inhibitor of NF-κBα (IκBα) phosphorylation and degradation, an inhibitor of NF-κB. Furthermore, an inhibitor of the IκBα kinase γ or NF-κB essential modulator, the regulatory component of the IKK complex, demonstrated that the NF-κB signalling pathway is mandatory for osteoclastogenesis induced by RANKL.
Conclusions and implications: Together, these data suggest that thiocolchicoside significantly suppressed osteoclastogenesis induced by RANKL and tumour cells via the NF-κB signalling pathway. Thus, thiocolchicoside, a drug that has been used for almost half a century to treat muscle pain, may also be considered as a new treatment for bone loss.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


Comment in
-
Thiocolchicoside a semi-synthetic derivative of the Glory Lily: a new weapon to fight metastatic bone resorption?Br J Pharmacol. 2012 Apr;165(7):2124-6. doi: 10.1111/j.1476-5381.2011.01792.x. Br J Pharmacol. 2012. PMID: 22122264 Free PMC article.
References
-
- Abu-Amer Y, Dowdy SF, Ross FP, Clohisy JC, Teitelbaum SL. TAT fusion proteins containing tyrosine 42-deleted IkappaBalpha arrest osteoclastogenesis. J Biol Chem. 2001;276:30499–30503. - PubMed
-
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. - PubMed
-
- Bauer DC, Orwoll ES, Fox KM, Vogt TM, Lane NE, Hochberg MC, et al. Aspirin and NSAID use in older women: effect on bone mineral density and fracture risk. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11:29–35. - PubMed
-
- Bharti AC, Aggarwal BB. Ranking the role of RANK ligand in apoptosis. Apoptosis. 2004;9:677–690. - PubMed
-
- Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004a;103:3175–3184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

