The metabolism and pharmacokinetics of phospho-sulindac (OXT-328) and the effect of difluoromethylornithine
- PMID: 21955327
- PMCID: PMC3413853
- DOI: 10.1111/j.1476-5381.2011.01705.x
The metabolism and pharmacokinetics of phospho-sulindac (OXT-328) and the effect of difluoromethylornithine
Abstract
Background and purpose: Phospho-sulindac (PS; OXT-328) prevents colon cancer in mice, especially when combined with difluoromethylornithine (DFMO). Here, we explored its metabolism and pharmacokinetics.
Experimental approach: PS metabolism was studied in cultured cells, liver microsomes and cytosol, intestinal microsomes and in mice. Pharmacokinetics and biodistribution of PS were studied in mice.
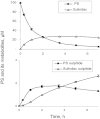
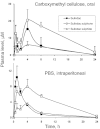
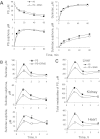
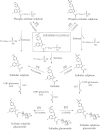
Key results: PS undergoes reduction and oxidation yielding PS sulphide and PS sulphone; is hydrolysed releasing sulindac, which generates sulindac sulphide (SSide) and sulindac sulphone (SSone), all of which are glucuronidated. Liver and intestinal microsomes metabolized PS extensively but cultured cells converted only 10% of it to PS sulphide and PS sulphone. In mice, oral PS is rapidly absorbed, metabolized and distributed to the blood and other tissues. PS survives only partially intact in blood; of its three major metabolites (sulindac, SSide and SSone), sulindac has the highest C(max) and SSone the highest t(1/2) ; their AUC(0-24h) are similar. Compared with conventional sulindac, PS generated more SSone but less SSide, which may contribute to the safety of PS. In the gastroduodenal wall of mice, 71% of PS was intact; sulindac, SSide and SSone together accounted for <30% of the total. This finding may explain the lack of gastrointestinal toxicity by PS. DFMO had no effect on PS metabolism but significantly reduced drug level in mouse plasma and other tissues.
Conclusions and implications: Our findings establish the metabolism of PS define its pharmacokinetics and biodistribution, describe its interactions with DFMO and largely explain its gastrointestinal safety.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures




Similar articles
-
Phospho-sulindac (OXT-328) combined with difluoromethylornithine prevents colon cancer in mice.Cancer Prev Res (Phila). 2011 Jul;4(7):1052-60. doi: 10.1158/1940-6207.CAPR-11-0067. Epub 2011 Apr 4. Cancer Prev Res (Phila). 2011. PMID: 21464038 Free PMC article.
-
Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice.Gastroenterology. 2010 Oct;139(4):1320-32. doi: 10.1053/j.gastro.2010.06.044. Epub 2010 Jun 20. Gastroenterology. 2010. PMID: 20600034 Free PMC article.
-
The ocular pharmacokinetics and biodistribution of phospho-sulindac (OXT-328) formulated in nanoparticles: Enhanced and targeted tissue drug delivery.Int J Pharm. 2019 Feb 25;557:273-279. doi: 10.1016/j.ijpharm.2018.12.057. Epub 2018 Dec 28. Int J Pharm. 2019. PMID: 30597269
-
Clinical pharmacokinetics of sulindac. A dynamic old drug.Clin Pharmacokinet. 1997 Jun;32(6):437-59. doi: 10.2165/00003088-199732060-00002. Clin Pharmacokinet. 1997. PMID: 9195115 Review.
-
Polyamines as mediators of APC-dependent intestinal carcinogenesis and cancer chemoprevention.Essays Biochem. 2009 Nov 4;46:111-24. doi: 10.1042/bse0460008. Essays Biochem. 2009. PMID: 20095973 Free PMC article. Review.
Cited by
-
NSAIDs and Colorectal Cancer Control: Promise and Challenges.Curr Pharmacol Rep. 2015 Oct 1;1(5):295-301. doi: 10.1007/s40495-015-0042-x. Epub 2015 May 14. Curr Pharmacol Rep. 2015. PMID: 26688785 Free PMC article.
-
A Second WNT for Old Drugs: Drug Repositioning against WNT-Dependent Cancers.Cancers (Basel). 2016 Jul 14;8(7):66. doi: 10.3390/cancers8070066. Cancers (Basel). 2016. PMID: 27429001 Free PMC article. Review.
-
Phospho-NSAIDs have enhanced efficacy in mice lacking plasma carboxylesterase: implications for their clinical pharmacology.Pharm Res. 2015 May;32(5):1663-75. doi: 10.1007/s11095-014-1565-2. Epub 2014 Nov 13. Pharm Res. 2015. PMID: 25392229 Free PMC article.
-
The evolving role of nonsteroidal anti-inflammatory drugs in colon cancer prevention: a cause for optimism.J Pharmacol Exp Ther. 2015 Apr;353(1):2-8. doi: 10.1124/jpet.114.220806. J Pharmacol Exp Ther. 2015. PMID: 25589413 Free PMC article. Review.
-
Phospho-sulindac (OXT-328) inhibits the growth of human lung cancer xenografts in mice: enhanced efficacy and mitochondria targeting by its formulation in solid lipid nanoparticles.Pharm Res. 2012 Nov;29(11):3090-101. doi: 10.1007/s11095-012-0801-x. Epub 2012 Jun 22. Pharm Res. 2012. PMID: 22723123 Free PMC article.
References
-
- Acosta EJ, Harwell JH, Sabatini DA. Self-assembly in linker-modified microemulsions. J Colloid Interface Sci. 2004;274:652–664. - PubMed
-
- Anders MW, Ratnayake JH, Hanna PE, Fuchs JA. Thioredoxin-dependent sulfoxide reduction by rat renal cytosol. Drug Metab Dispos. 1981;9:307–310. - PubMed
-
- Babbar N, Ignatenko NA, Casero RA, Jr, Gerner EW. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem. 2003;278:47762–47775. - PubMed
-
- Baron JA. Aspirin and NSAIDs for the prevention of colorectal cancer. Recent Results Cancer Res. 2009;181:223–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

