Lack of adaptation to human tetherin in HIV-1 group O and P
- PMID: 21955466
- PMCID: PMC3192746
- DOI: 10.1186/1742-4690-8-78
Lack of adaptation to human tetherin in HIV-1 group O and P
Abstract
Background: HIV-1 viruses are categorized into four distinct groups: M, N, O and P. Despite the same genomic organization, only the group M viruses are responsible for the world-wide pandemic of AIDS, suggesting better adaptation to human hosts. Previously, it has been reported that the group M Vpu protein is capable of both down-modulating CD4 and counteracting BST-2/tetherin restriction, while the group O Vpu cannot antagonize tetherin. This led us to investigate if group O, and the related group P viruses, possess functional anti-tetherin activities in Vpu or another viral protein, and to further map the residues required for group M Vpu to counteract human tetherin.
Results: We found a lack of activity against human tetherin for both the Vpu and Nef proteins from group O and P viruses. Furthermore, we found no evidence of anti-human tetherin activity in a fully infectious group O proviral clone, ruling out the possibility of an alternative anti-tetherin factor in this virus. Interestingly, an activity against primate tetherins was retained in the Nef proteins from both a group O and a group P virus. By making chimeras between a functional group M and non-functional group O Vpu protein, we were able to map the first 18 amino acids of group M Vpu as playing an essential role in the ability of the protein to antagonize human tetherin. We further demonstrated the importance of residue alanine-18 for the group M Vpu activity. This residue lies on a diagonal face of conserved alanines in the TM domain of the protein, and is necessary for specific Vpu-tetherin interactions.
Conclusions: The absence of human specific anti-tetherin activities in HIV-1 group O and P suggests a failure of these viruses to adapt to human hosts, which may have limited their spread.
Figures
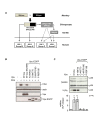
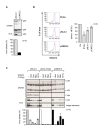
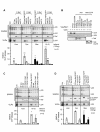
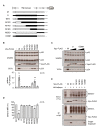
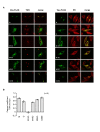
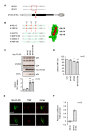
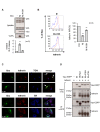

Similar articles
-
HIV-1 Group P is unable to antagonize human tetherin by Vpu, Env or Nef.Retrovirology. 2011 Dec 15;8:103. doi: 10.1186/1742-4690-8-103. Retrovirology. 2011. PMID: 22171785 Free PMC article.
-
βTrCP is Required for HIV-1 Vpu Modulation of CD4, GaLV Env, and BST-2/Tetherin.Viruses. 2018 Oct 19;10(10):573. doi: 10.3390/v10100573. Viruses. 2018. PMID: 30347660 Free PMC article.
-
Functional antagonism of rhesus macaque and chimpanzee BST-2 by HIV-1 Vpu is mediated by cytoplasmic domain interactions.J Virol. 2013 Dec;87(24):13825-36. doi: 10.1128/JVI.02567-13. Epub 2013 Oct 9. J Virol. 2013. PMID: 24109238 Free PMC article.
-
Transmembrane interactions of HIV-1 Vpu and tetherin.Curr HIV Res. 2012 Jun;10(4):292-7. doi: 10.2174/157016212800792450. Curr HIV Res. 2012. PMID: 22524177 Review.
-
Tetherin/BST-2: Restriction Factor or Immunomodulator?Curr HIV Res. 2016;14(3):235-46. doi: 10.2174/1570162x14999160224102752. Curr HIV Res. 2016. PMID: 26957198 Free PMC article. Review.
Cited by
-
The role of BST-2/Tetherin in host protection and disease manifestation.Immun Inflamm Dis. 2015 Dec 7;4(1):4-23. doi: 10.1002/iid3.92. eCollection 2016 Mar. Immun Inflamm Dis. 2015. PMID: 27042298 Free PMC article. Review.
-
Preadaptation of Simian Immunodeficiency Virus SIVsmm Facilitated Env-Mediated Counteraction of Human Tetherin by Human Immunodeficiency Virus Type 2.J Virol. 2018 Aug 29;92(18):e00276-18. doi: 10.1128/JVI.00276-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976668 Free PMC article.
-
Efficient Vpu-Mediated Tetherin Antagonism by an HIV-1 Group O Strain.J Virol. 2017 Feb 28;91(6):e02177-16. doi: 10.1128/JVI.02177-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077643 Free PMC article.
-
Experimental Adaptive Evolution of Simian Immunodeficiency Virus SIVcpz to Pandemic Human Immunodeficiency Virus Type 1 by Using a Humanized Mouse Model.J Virol. 2018 Jan 30;92(4):e01905-17. doi: 10.1128/JVI.01905-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29212937 Free PMC article.
-
Targeting frameshifting in the human immunodeficiency virus.Expert Opin Ther Targets. 2012 Mar;16(3):249-58. doi: 10.1517/14728222.2012.665879. Expert Opin Ther Targets. 2012. PMID: 22404160 Free PMC article. Review.
References
-
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli JC. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. - DOI - PMC - PubMed
-
- Sauter D, Schindler M, Specht A, Landford WN, Münch J, Kim KA, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, Takehisa J, Ogando Y, Ochsenbauer C, Kappes JC, Ayouba A, Peeters M, Learn GH, Shaw G, Sharp PM, Bieniasz PD, Hahn BH, Hatziioannou T, Kirchhoff F. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6:409–421. doi: 10.1016/j.chom.2009.10.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

