3D cell entrapment in crosslinked thiolated gelatin-poly(ethylene glycol) diacrylate hydrogels
- PMID: 21955690
- PMCID: PMC3282186
- DOI: 10.1016/j.biomaterials.2011.09.031
3D cell entrapment in crosslinked thiolated gelatin-poly(ethylene glycol) diacrylate hydrogels
Abstract
The combined use of natural ECM components and synthetic materials offers an attractive alternative to fabricate hydrogel-based tissue engineering scaffolds to study cell-matrix interactions in three-dimensions (3D). A facile method was developed to modify gelatin with cysteine via a bifunctional PEG linker, thus introducing free thiol groups to gelatin chains. A covalently crosslinked gelatin hydrogel was fabricated using thiolated gelatin and poly(ethylene glycol) diacrylate (PEGdA) via thiol-ene reaction. Unmodified gelatin was physically incorporated in a PEGdA-only matrix for comparison. We sought to understand the effect of crosslinking modality on hydrogel physicochemical properties and the impact on 3D cell entrapment. Compared to physically incorporated gelatin hydrogels, covalently crosslinked gelatin hydrogels displayed higher maximum weight swelling ratio (Q(max)), higher water content, significantly lower cumulative gelatin dissolution up to 7 days, and lower gel stiffness. Furthermore, fibroblasts encapsulated within covalently crosslinked gelatin hydrogels showed extensive cytoplasmic spreading and the formation of cellular networks over 28 days. In contrast, fibroblasts encapsulated in the physically incorporated gelatin hydrogels remained spheroidal. Hence, crosslinking ECM protein with synthetic matrix creates a stable scaffold with tunable mechanical properties and with long-term cell anchorage points, thus supporting cell attachment and growth in the 3D environment.
Published by Elsevier Ltd.
Figures



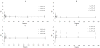


 ); loss modulus G″ at R.T. (○), 37 °C (◊). *, significantly different compared to corresponding nonswollen formulation with p < 0.05.
); loss modulus G″ at R.T. (○), 37 °C (◊). *, significantly different compared to corresponding nonswollen formulation with p < 0.05.
 ), Gcys10P340015 and G10P340015 (●), Gcys10P340020 and G10P340020 (
), Gcys10P340015 and G10P340015 (●), Gcys10P340020 and G10P340020 (
 ); loss modulus G″ of Gcys10P340010 and G10P340010 (◊), Gcys10P340015 and G10P340015 (○), Gcys10P340020 and G10P340020 (□). Complex shear moduli of covalently crosslinked Gel-PEG-Cys hydrogels (B) and physically incorporated gelatin hydrogels (D). *, significantly different compared to corresponding nonswollen formulation with p < 0.05.
); loss modulus G″ of Gcys10P340010 and G10P340010 (◊), Gcys10P340015 and G10P340015 (○), Gcys10P340020 and G10P340020 (□). Complex shear moduli of covalently crosslinked Gel-PEG-Cys hydrogels (B) and physically incorporated gelatin hydrogels (D). *, significantly different compared to corresponding nonswollen formulation with p < 0.05.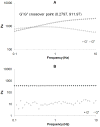
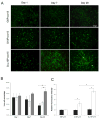
 ) at various culture times. (C) Fibroblasts proliferation in 3D conditions at day 1 (■) and day 7 (□) of culture. Fluorescence intensities of cells entrapped in covalently crosslinked Gcys10P340015 hydrogels and physically incorporated G10P340015 hydrogels were normalized to that of PEGdA-only hydrogel G0P340015. *, significantly different with p < 0.05.
) at various culture times. (C) Fibroblasts proliferation in 3D conditions at day 1 (■) and day 7 (□) of culture. Fluorescence intensities of cells entrapped in covalently crosslinked Gcys10P340015 hydrogels and physically incorporated G10P340015 hydrogels were normalized to that of PEGdA-only hydrogel G0P340015. *, significantly different with p < 0.05.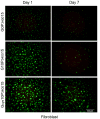
Similar articles
-
Synthesis of stiffness-tunable and cell-responsive Gelatin-poly(ethylene glycol) hydrogel for three-dimensional cell encapsulation.J Biomed Mater Res A. 2016 Oct;104(10):2401-11. doi: 10.1002/jbm.a.35779. Epub 2016 May 30. J Biomed Mater Res A. 2016. PMID: 27170015
-
Thiol-ene-based biological/synthetic hybrid biomatrix for 3-D living cell culture.Acta Biomater. 2012 Jul;8(7):2504-16. doi: 10.1016/j.actbio.2012.03.049. Epub 2012 Apr 5. Acta Biomater. 2012. PMID: 22484717 Free PMC article.
-
Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds.Biomaterials. 2014 Feb;35(6):1845-56. doi: 10.1016/j.biomaterials.2013.11.009. Epub 2013 Dec 5. Biomaterials. 2014. PMID: 24314597
-
Recent Developments in Thiolated Polymeric Hydrogels for Tissue Engineering Applications.Tissue Eng Part B Rev. 2018 Feb;24(1):66-74. doi: 10.1089/ten.TEB.2016.0442. Epub 2017 Aug 24. Tissue Eng Part B Rev. 2018. PMID: 28726576 Review.
-
Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering.Biomaterials. 2010 Jun;31(17):4639-56. doi: 10.1016/j.biomaterials.2010.02.044. Epub 2010 Mar 19. Biomaterials. 2010. PMID: 20303169 Free PMC article. Review.
Cited by
-
Optimized hyaluronic acid-hydrogel design and culture conditions for preservation of mesenchymal stem cell properties.Tissue Eng Part C Methods. 2013 Apr;19(4):288-98. doi: 10.1089/ten.TEC.2012.0144. Epub 2012 Oct 25. Tissue Eng Part C Methods. 2013. PMID: 22992013 Free PMC article.
-
A Pyk2 inhibitor incorporated into a PEGDA-gelatin hydrogel promotes osteoblast activity and mineral deposition.Biomed Mater. 2019 Feb 27;14(2):025015. doi: 10.1088/1748-605X/aafffa. Biomed Mater. 2019. PMID: 30658347 Free PMC article.
-
Microstructured Elastomer-PEG Hydrogels via Kinetic Capture of Aqueous Liquid-Liquid Phase Separation.Adv Sci (Weinh). 2018 Mar 12;5(6):1701010. doi: 10.1002/advs.201701010. eCollection 2018 Jun. Adv Sci (Weinh). 2018. PMID: 29938180 Free PMC article.
-
A Versatile Biosynthetic Hydrogel Platform for Engineering of Tissue Analogues.Adv Healthc Mater. 2019 Oct;8(19):e1900979. doi: 10.1002/adhm.201900979. Epub 2019 Aug 12. Adv Healthc Mater. 2019. PMID: 31402634 Free PMC article.
-
Preparation and characterization of electrospun PLCL/Poloxamer nanofibers and dextran/gelatin hydrogels for skin tissue engineering.PLoS One. 2014 Nov 18;9(11):e112885. doi: 10.1371/journal.pone.0112885. eCollection 2014. PLoS One. 2014. PMID: 25405611 Free PMC article.
References
-
- Chen G, Ushida T, Tateishi T. Scaffold design for tissue engineering. Macromol Biosci. 2002;2:67–77.
-
- Bott K, Upton Z, Schrobback K, Ehrbar M, Hubbel JA, Lutolf MP, et al. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials. 2010;31:8454–64. - PubMed
-
- Cushing MC, Anseth KS. Materials science. Hydrogel cell cultures. Science. 2007;316:1133–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

