Cooperation of p40(phox) with p47(phox) for Nox2-based NADPH oxidase activation during Fcγ receptor (FcγR)-mediated phagocytosis: mechanism for acquisition of p40(phox) phosphatidylinositol 3-phosphate (PI(3)P) binding
- PMID: 21956105
- PMCID: PMC3220462
- DOI: 10.1074/jbc.M111.237289
Cooperation of p40(phox) with p47(phox) for Nox2-based NADPH oxidase activation during Fcγ receptor (FcγR)-mediated phagocytosis: mechanism for acquisition of p40(phox) phosphatidylinositol 3-phosphate (PI(3)P) binding
Abstract
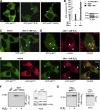
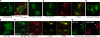
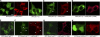
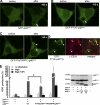
During activation of the phagocyte (Nox2-based) NADPH oxidase, the cytoplasmic Phox complex (p47(phox)-p67(phox)-p40(phox)) translocates and associates with the membrane-spanning flavocytochrome b(558). It is unclear where (in cytoplasm or on membranes), when (before or after assembly), and how p40(phox) acquires its PI(3)P-binding capabilities. We demonstrated that in addition to conformational changes induced by H(2)O(2) in the cytoplasm, p40(phox) acquires PI(3)P-binding through direct or indirect membrane targeting. We also found that p40(phox) is essential when p47(phox) is partially phosphorylated during FcγR-mediated oxidase activation; however, p40(phox) is less critical when p47(phox) is adequately phosphorylated, using phosphorylation-mimicking mutants in HEK293(Nox2/FcγRIIa) and RAW264.7(p40/p47KD) cells. Moreover, PI binding to p47(phox) is less important when the autoinhibitory PX-PB1 domain interaction in p40(phox) is disrupted or when p40(phox) is targeted to membranes. Furthermore, we suggest that high affinity PI(3)P binding of the p40(phox) PX domain is critical during its accumulation on phagosomes, even when masked by the PB1 domain in the resting state. Thus, in addition to mechanisms for directly acquiring PI(3)P binding in the cytoplasm by H(2)O(2), p40(phox) can acquire PI(3)P binding on targeted membranes in a p47(phox)-dependent manner and functions both as a "carrier" of the cytoplasmic Phox complex to phagosomes and an "adaptor" of oxidase assembly on phagosomes in cooperation with p47(phox), using positive feedback mechanisms.
Figures

Similar articles
-
A regulated adaptor function of p40phox: distinct p67phox membrane targeting by p40phox and by p47phox.Mol Biol Cell. 2007 Feb;18(2):441-54. doi: 10.1091/mbc.e06-08-0731. Epub 2006 Nov 22. Mol Biol Cell. 2007. PMID: 17122360 Free PMC article.
-
Fc gamma R-stimulated activation of the NADPH oxidase: phosphoinositide-binding protein p40phox regulates NADPH oxidase activity after enzyme assembly on the phagosome.Blood. 2008 Nov 1;112(9):3867-77. doi: 10.1182/blood-2007-11-126029. Epub 2008 Aug 18. Blood. 2008. PMID: 18711001 Free PMC article.
-
The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcgammaIIA receptor-induced phagocytosis.J Exp Med. 2006 Aug 7;203(8):1915-25. doi: 10.1084/jem.20052085. Epub 2006 Jul 31. J Exp Med. 2006. PMID: 16880255 Free PMC article.
-
Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task.Small GTPases. 2014;5:e27952. doi: 10.4161/sgtp.27952. Epub 2014 Mar 5. Small GTPases. 2014. PMID: 24598074 Free PMC article. Review.
-
NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits.Eur J Clin Invest. 2018 Nov;48 Suppl 2:e12951. doi: 10.1111/eci.12951. Epub 2018 Jun 3. Eur J Clin Invest. 2018. PMID: 29757466 Review.
Cited by
-
Regulation of Neutrophil NADPH Oxidase, NOX2: A Crucial Effector in Neutrophil Phenotype and Function.Front Cell Dev Biol. 2022 Jul 14;10:945749. doi: 10.3389/fcell.2022.945749. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35912108 Free PMC article. Review.
-
Enhanced generation of reactive oxygen species by interferon-γ may have contributed to successful treatment of invasive pulmonary aspergillosis in a patient with chronic granulomatous disease.Int J Hematol. 2013 Apr;97(4):505-10. doi: 10.1007/s12185-013-1315-y. Epub 2013 Mar 24. Int J Hematol. 2013. PMID: 23526099
-
Carcinogenesis and Reactive Oxygen Species Signaling: Interaction of the NADPH Oxidase NOX1-5 and Superoxide Dismutase 1-3 Signal Transduction Pathways.Antioxid Redox Signal. 2019 Jan 20;30(3):443-486. doi: 10.1089/ars.2017.7268. Epub 2018 Nov 22. Antioxid Redox Signal. 2019. PMID: 29478325 Free PMC article. Review.
-
RAB5c controls the assembly of non-canonical autophagy machinery to promote phagosome maturation and microbicidal function of macrophages.bioRxiv [Preprint]. 2025 Mar 28:2025.03.25.645097. doi: 10.1101/2025.03.25.645097. bioRxiv. 2025. PMID: 40196584 Free PMC article. Preprint.
-
Enriched conditioning expands the regenerative ability of sensory neurons after spinal cord injury via neuronal intrinsic redox signaling.Nat Commun. 2020 Dec 21;11(1):6425. doi: 10.1038/s41467-020-20179-z. Nat Commun. 2020. PMID: 33349630 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

