Mechanical regulation of glycogen synthase kinase 3β (GSK3β) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein
- PMID: 21956113
- PMCID: PMC3234768
- DOI: 10.1074/jbc.M111.265330
Mechanical regulation of glycogen synthase kinase 3β (GSK3β) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein
Abstract
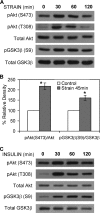
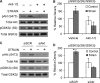
Mechanical signals can inactivate glycogen synthase kinase 3β (GSK3β), resulting in stabilization of β-catenin. This signaling cascade is necessary for the inhibition of adipogenesis in mesenchymal stem cells (MSC) that is produced by a daily strain regimen. We investigated whether Akt is the mechanically activated kinase responsible for phosphorylation and inactivation of GSK3β in MSC. Mechanical strain (2% magnitude, 0.17 Hz) induced phosphorylation of Akt at Ser-473 and Thr-308 in parallel with phosphorylation of GSK3β at Ser-9. Inhibiting Akt (Akt1/2 kinase inhibitor treatment or Akt knockdown) prevented strain-induced phosphorylation of GSK3β at Ser-9. Inhibition of PI3K prevented Thr-308 phosphorylation, but strain-induced Ser-473 phosphorylation was measurable and induced phosphorylation of GSK3β, suggesting that Ser-473 phosphorylation is sufficient for the downstream mechanoresponse. As Rictor/mTORC2 (mammalian target of rapamycin complex 2) is known to transduce phosphorylation of Akt at Ser-473 by insulin, we investigated whether it contributes to strain-induced Ser-473 phosphorylation. Phosphorylation of Ser-473 by both mechanical and insulin treatment in MSC was prevented by the mTOR inhibitor KU0063794. When mTORC2 was blocked, mechanical GSK3β inactivation was prevented, whereas insulin inhibition of GSK3β was still measured in the absence of Ser-473 phosphorylation, presumably through phosphorylation of Akt at Thr-308. In sum, mechanical input initiates a signaling cascade that is uniquely dependent on mTORC2 activation and phosphorylation of Akt at Ser-473, an effect sufficient to cause inactivation of GSK3β. Thus, mechanical regulation of GSK3β downstream of Akt is dependent on phosphorylation of Akt at Ser-473 in a manner distinct from that of growth factors. As such, Akt reveals itself to be a pleiotropic signaling molecule whose downstream targets are differentially regulated depending upon the nature of the activating input.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

