MicroRNA 135 regulates HOXA10 expression in endometriosis
- PMID: 21956427
- PMCID: PMC3232619
- DOI: 10.1210/jc.2011-1231
MicroRNA 135 regulates HOXA10 expression in endometriosis
Abstract
Context: Homeo box A10 (HOXA10) regulates endometrial receptivity and its expression is decreased in women with endometriosis. Although sex steroids regulate HOXA10, these hormones are unaltered in endometriosis. We hypothesized a role for microRNA in the regulation of HOXA10.
Objective: MicroRNA 135a and -b are small noncoding RNA with predicted targets that include HOXA10. We evaluated miR135a/b expression and HOXA10 regulation in endometrium from subjects with and without endometriosis.
Design: The design of the study was the measurement of miR135a/b expression by quantitative PCR and in vitro analysis of HOXA10 regulation.
Setting: The study was conducted at a university medical center.
Patients: Patients included 50 controls and 32 women with endometriosis.
Interventions: Study interventions included endometrial biopsies and in vitro transfection.
Main outcome measures: miR135a/b and HOXA10 expression were measured in the study.
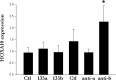
Results: All endometrial samples expressed miR135a and -b. miR135a expression in controls was increased during the proliferative phase, decreased at the time of ovulation, and increased during the luteal phase. Subjects with endometriosis had 3-fold higher expression of miR135a in the proliferative phase than controls. miR135b showed less variation across the menstrual cycle; however, it was significantly increased in women with endometriosis in the proliferative and secretory phases. HOXA10 expression was simultaneously repressed in the endometrium of women with endometriosis. Transfection of endometrial stromal cells with mir135a/b or miR135a/b inhibitors resulted in the altered expression of HOXA10 mRNA and protein. miR135a or -b decreased luciferase expression driven by the HOXA10 3' untranslated region containing the miR135 binding site. miR135a regulation of HOXA10 was absent in MCF-7 cells, demonstrating cell specificity.
Conclusions: HOXA10 was aberrantly regulated in the endometrium of women with endometriosis by both miR135a and miR135b. Increased microRNA expression likely suppresses genes required for implantation.
Figures





References
-
- Sampson J. 1927. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:422–469
-
- Du H, Taylor HS. 2007. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 25:2082–2086 - PubMed
-
- de Ziegler D, Borghese B, Chapron C. 2010. Endometriosis and infertility: pathophysiology and management. Lancet 376:730–738 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

